- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE IGCSE Biology: 复习笔记:1.1.3 Pressure & Temperature in Gases
CIE IGCSE Biology: 复习笔记:1.1.3 Pressure & Temperature in Gases
Pressure & Temperature in Gases
- A change in temperature or pressure affects the volume of gases
- As the air inside a hot air balloon is heated up, it expands and the balloon gets bigger
- This is because the volume of a gas increases as its temperature increases
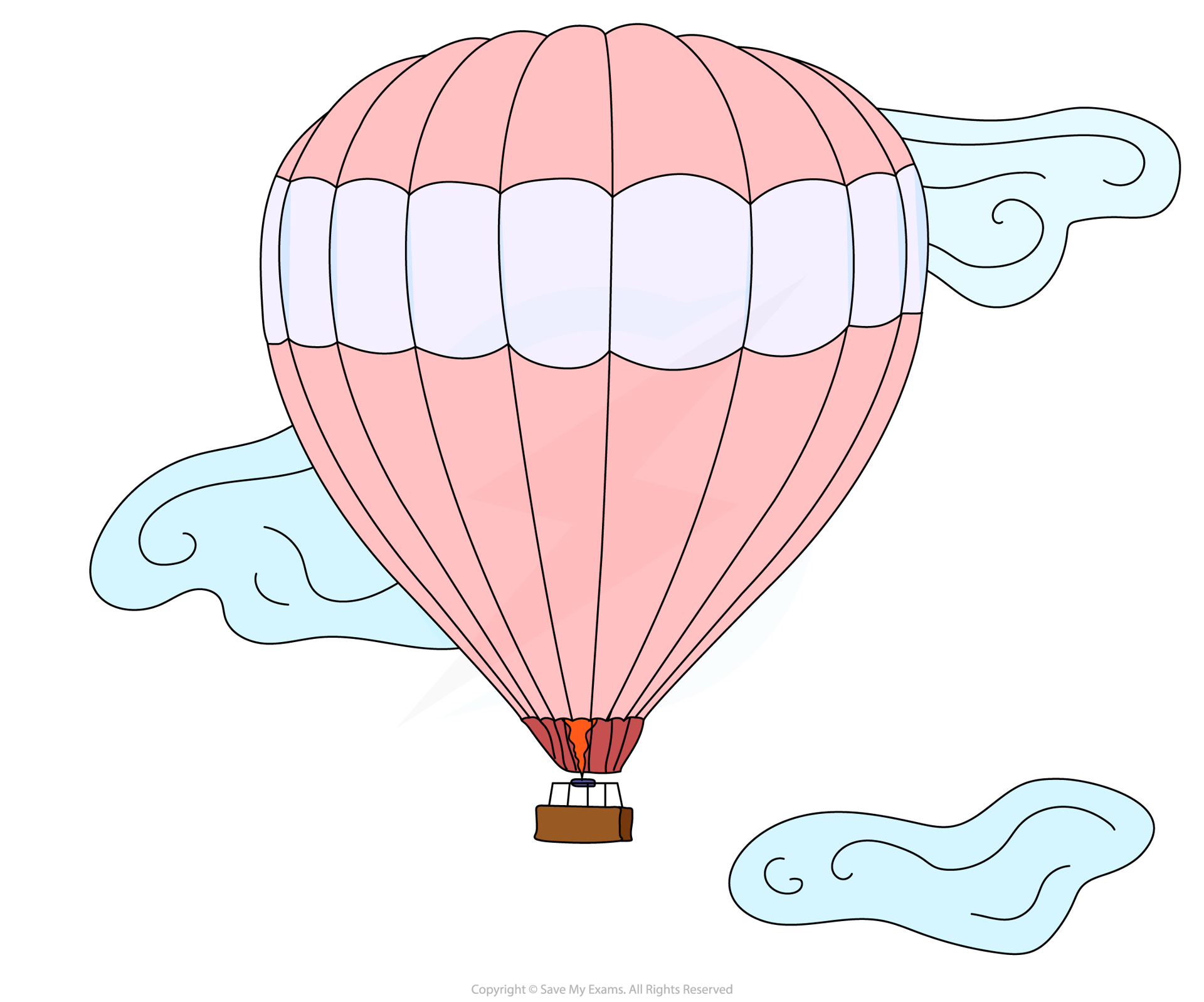
As temperature increases gas volume increases. The density decreases as the volume increases so the balloon rises.
- If you have a gas stored inside a container that is squeezed, the pressure increases as you decrease the volume
- This is what happens in a bicycle pump
- As you compress the bicycle pump the high pressure allows you to inflate a tire
- You can feel the force of the high pressure if you put your finger on the end of the pump

Pressure increases as volume decreases in a bicycle pump
Gases & Kinetic Theory
EXTENDED
- Gaseous particles are in constant and random motion
- The pressure that gas creates inside a closed container is produced by the gaseous particles hitting the inside walls of the container
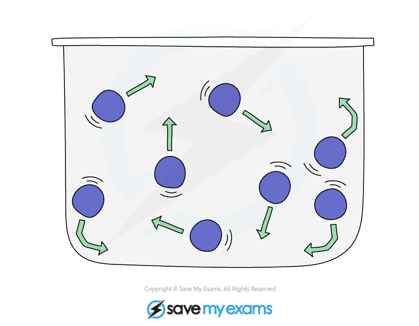
Moving particles of gas colliding with each other and the container walls
- An increase in temperature increases the kinetic energy of each particle, as the heat energy is transformed to kinetic energy, so they move faster
- As the temperature increases, the particles in the gas move faster, impacting the container's walls more frequently
- If the container walls are flexible and stretchy then the container will get bigger and bigger, just like the hot air balloon!
- If the container is made smaller, then the gas particles hit the wall more frequently
- So when there is a decrease in volume this causes an increase in gas pressure
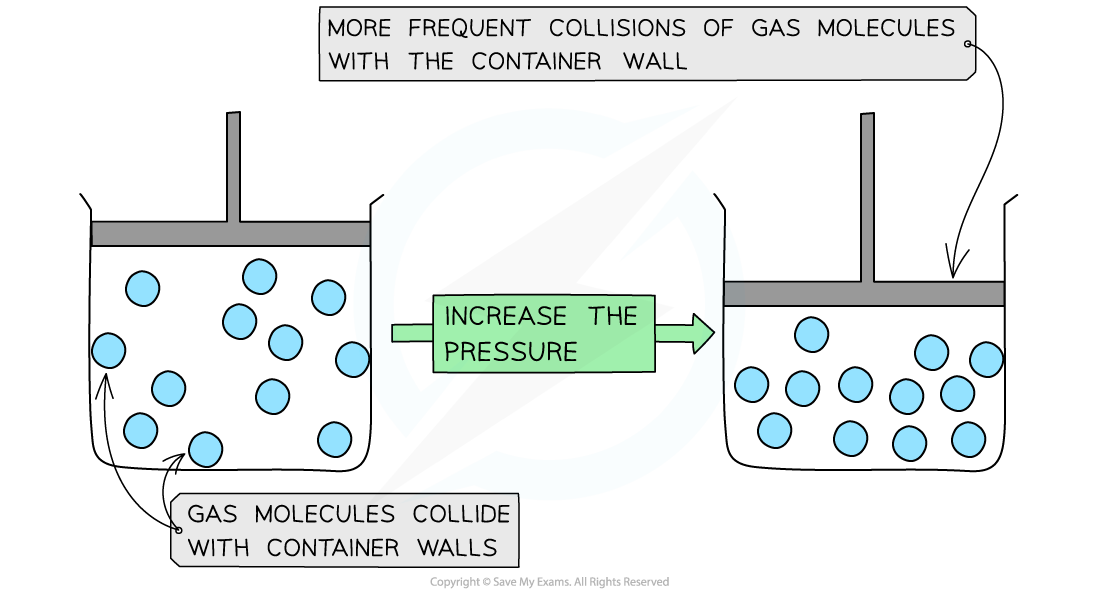
Molecules collide more frequently with the container walls when the pressure is increased
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





