- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
OCR A Level Biology:复习笔记2.2.16 Finding the Concentration of a Substance
Finding the Concentration of a Substance
- Benedict’s solution can be used to carry out a semi-quantitative test on a reducing sugar solution to determine the concentration of reducing sugar present in the sample
- It is important that an excess of Benedict’s solution is used so that there is more than enough copper (II) sulfate present to react with any sugar present
- The intensity of any colour change seen relates to the concentration of reducing sugar present in the sample
- A positive test is indicated along a spectrum of colour from green (low concentration) to brick-red (high concentration of reducing sugar present)
- A semi-quantitative test can be carried out by setting up standard solutions with known concentrations of reducing sugar (such as glucose)
- These solutions should be set up using a serial dilution of an existing stock solution
- Each solution is then treated in the same way: add the same volume of Benedict’s solution to each sample and heat in a water bath that has been boiled (ideally at the same temperature each time) for a set time (5 minutes or so) to allow colour changes to occur
- It is important to ensure that an excess of Benedict’s solution is used
- Any colour change observed for each solution of a known concentration in that time can be attributed to the concentration of reducing sugar present in that solution
- The same procedure is carried out on a sample with an unknown concentration of reducing sugar which is then compared to the stock solution colours to estimate the concentration of reducing sugar present
Producing serial dilutions
- Serial dilutions are created by taking a series of dilutions of a stock solution. The concentration decreases by the same quantity between each test tube
- They can either be ‘doubling dilutions’ (where the concentration is halved between each test tube) or a desired range (e.g. 0, 2, 4, 6, 8, 10 mmol dm-3)
- Serial dilutions are completed to create a standard to compare unknown concentrations against
- The comparison can be:
- Visual
- Measured through a calibration/standard curve
- Measured using a colorimeter
- They can be used when:
- Counting bacteria or yeast populations
- Determining unknown glucose, starch, protein concentrations
- The comparison can be:
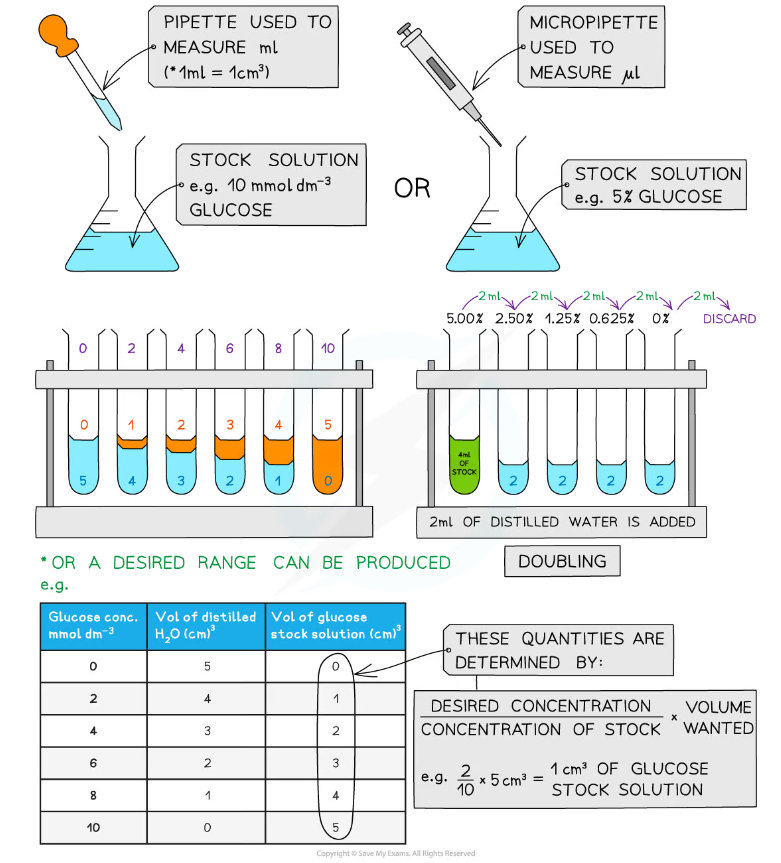
Making serial dilutions
Alterations
- It is also possible to standardise this test but instead of waiting a fixed amount of time for a range of colours to be observed, time how long it takes for the first colour change to occur (blue to green)
- The higher the concentration of reducing sugar in a sample, the less time it would take for a colour change to be observed
- To avoid issues with human interpretation of colour, a colorimeter could be used to measure the absorbance or transmission of light through the sugar solutions of known concentration to establish a range of values that an unknown sample can be compared against a calibration curve
Colorimeters
- A colorimeter is an instrument that beams a specific wavelength (colour) of light through a sample and measures how much of this light is absorbed (arbitrary units)
- They provide a quantitative measurement
- They contain different wavelengths or colour filters (depends on the model of colorimeter), so that a suitable colour can be shone through the sample and will not get absorbed. This colour will be the contrasting colour (eg. a red sample should have green light shone through)
- Remember that a sample will look red as that wavelength of light is being reflected but the other wavelengths will be absorbed
- Colorimeters must be calibrated before taking measurements
- This is completed by placing a blank into the colorimeter and taking a reference, it should read 0 (that is, no light is being absorbed)
- This step should be repeated periodically whilst taking measurements to ensure that the absorbance is still 0
- The results can then be used to plot a calibration or standard curve
- Absorbance/transmission of light against the known concentrations can be used
- Unknown concentrations can then be determined from this graph
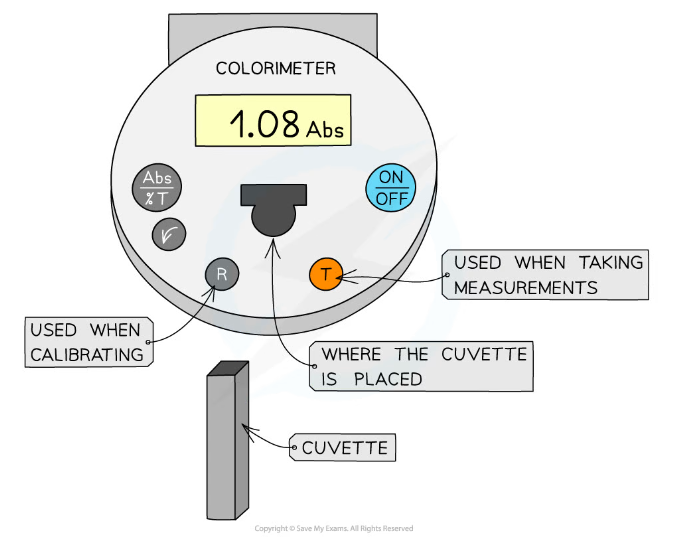
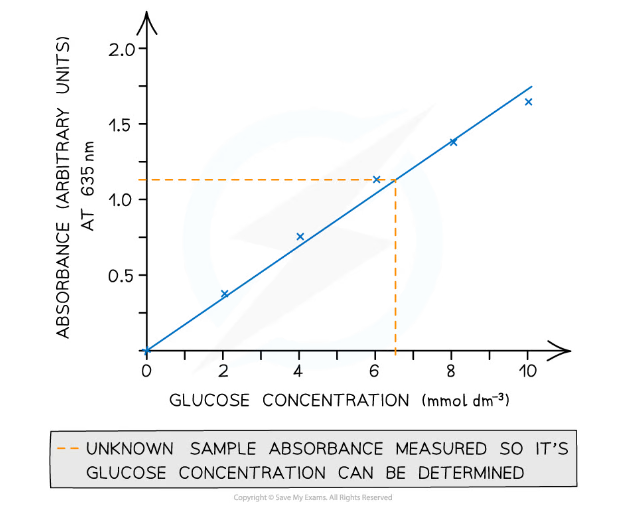
A colorimeter is used to obtain quantitative data that can be plotted to create a calibration curve to be used to find unknown concentrations
Biosensors
- Advances in technology mean there are now other methods of finding the concentration of a substance
- Biosensors are highly accurate analytical devices
- They use a catalyst to turn a biological response into an electrical signal
- The catalyst used is a very specific and stable enzyme
- Blood glucose biosensors are often used by diabetics so they can monitor and regulate their blood glucose levels
- Glucose oxidase is the enzyme used in these types of biosensors. It breaks down the glucose in the blood sample:
- Glucose oxidase uses FAD to oxidise glucose, forming FADH2
- FADH2 is then oxidised by the electrode in the device and this produces a current
- The current is a measurement of the glucose concentration
Using a blood glucose biosensor
- An individual produces a drop of blood on their finger using a pricker or a sterile lancet
- The blood is transferred onto a test strip
- The enzyme is located on the test strip
- The test strip is inserted into the biosensor meter
- A blood glucose reading is displayed as a digital figure
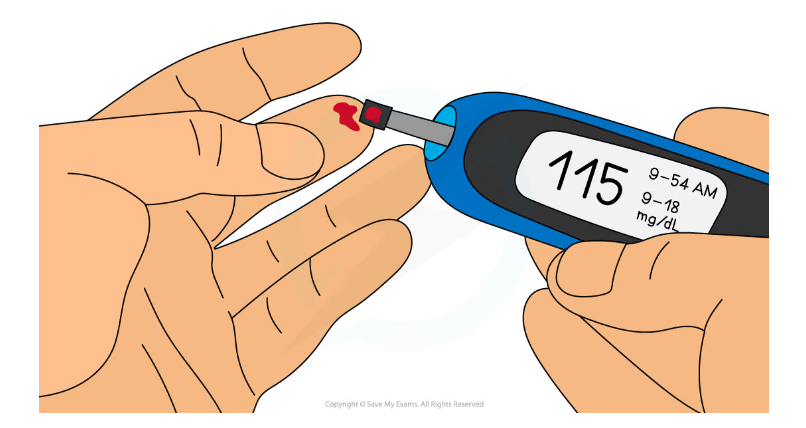
Blood glucose biosensors can allow diabetics to detect if their blood glucose concentration has become dangerously high or low
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





