- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
OCR A Level Biology:复习笔记2.2.5 Polysaccharides
Polysaccharides: Structure
- Starch, glycogen and cellulose are polysaccharides
- Polysaccharides are macromolecules (polymers) that are formed by many monosaccharides joined by glycosidic bonds in a condensation reaction to form chains
- These chains may be:
- Branched or unbranched
- Folded (making the molecule compact which is ideal for storage eg. starch and glycogen)
- Straight (making the molecules suitable to construct cellular structures e.g. cellulose) or coiled
Starch
- Starch is constructed from two different polysaccharides:
- Amylose (10 - 30% of starch)
- Unbranched helix-shaped chain with 1,4 glycosidic bonds between α-glucose molecules
- The helix shape enables it to be more compact and thus it is more resistant to digestion
- Amylopectin (70 - 90% of starch)
- 1,4 glycosidic bonds between α-glucose molecules but also 1,6 glycosidic bonds form between glucose molecules creating a branched molecule
- Amylose (10 - 30% of starch)
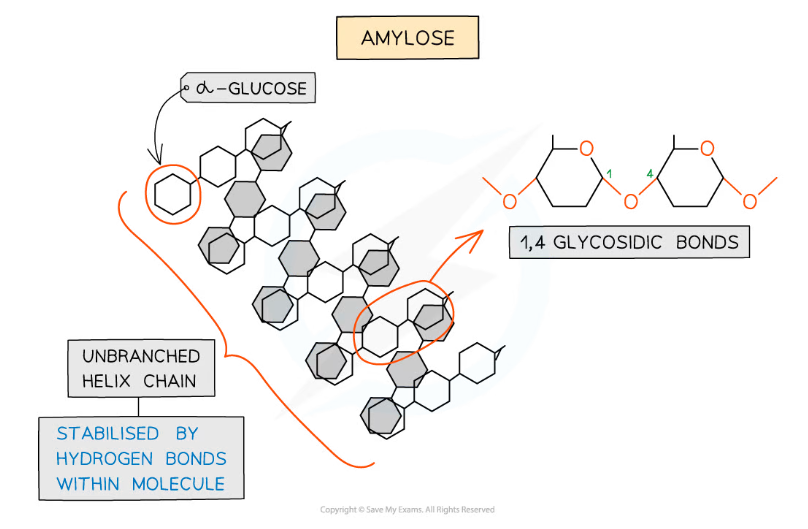
Amylose – one of the two polysaccharides that is used to form starch (the storage polysaccharide in plants)
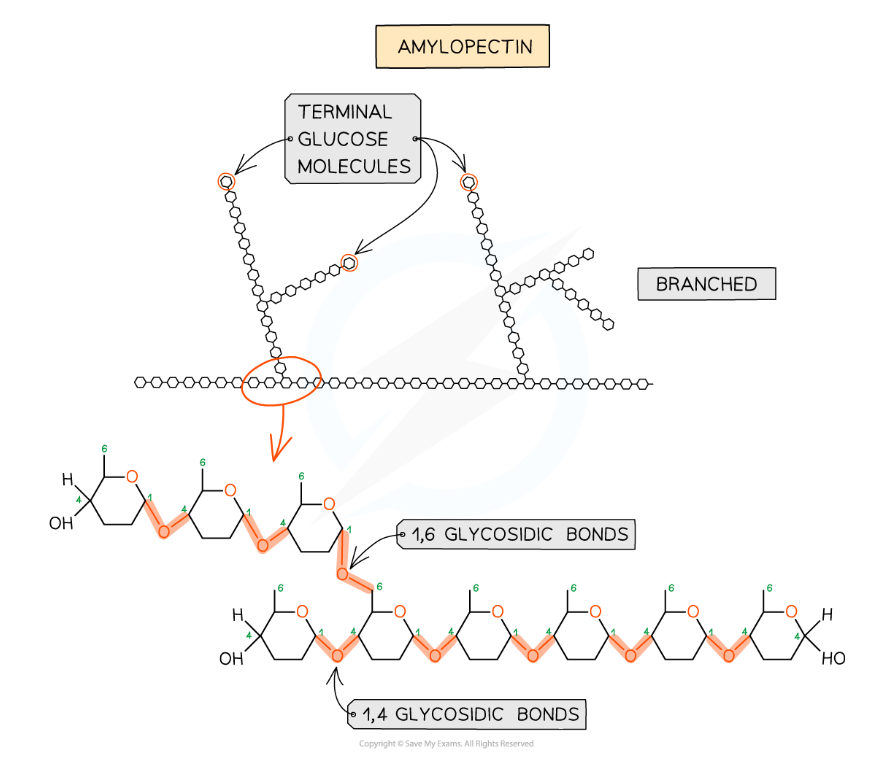
Amylopectin – one of the two polysaccharides that is used to form starch (the storage polysaccharide in plants)
Glycogen
- Glycogen is a polysaccharide found in animals
- It is made up of α-glucose molecules
- There are 1,4 glycosidic bonds between α-glucose molecules and also 1,6 glycosidic bonds between glucose molecules creating a branched molecule
- Glycogen has a similar structure to amylopectin but it has more branches
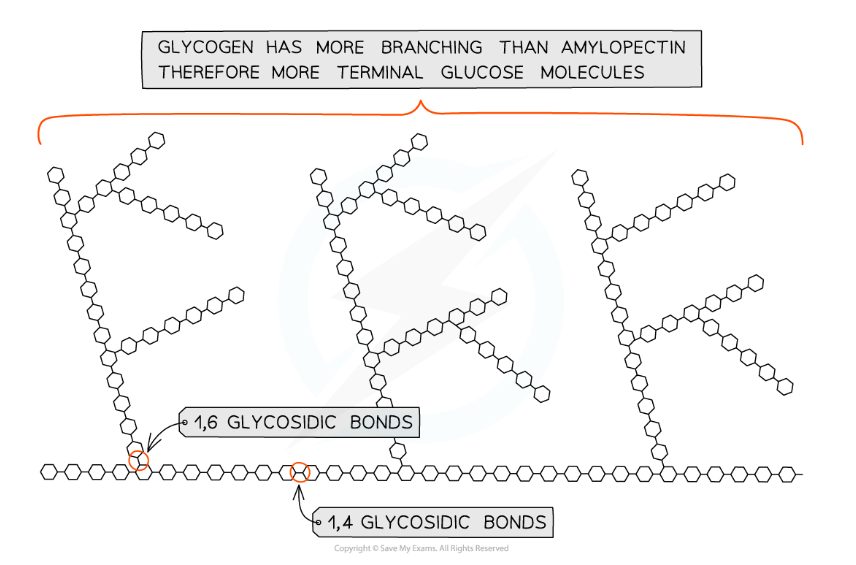
Glycogen, the highly branched molecule used as a storage polysaccharide in animals and fungi
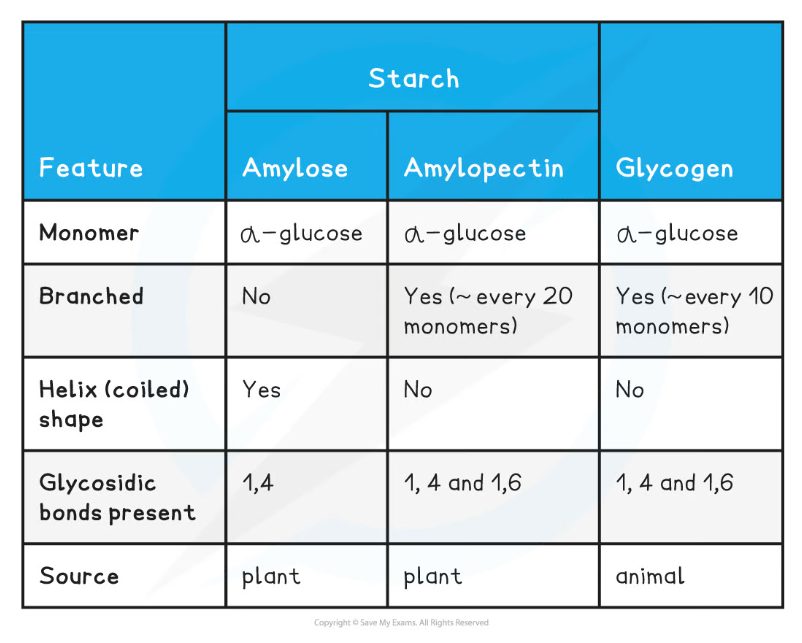
Summary of Storage Polysaccharides Table
Cellulose
- Cellulose is a polysaccharide found in plants
- It consists of long chains of β-glucose joined together by 1,4 glycosidic bonds
- β-glucose is an isomer of α-glucose, so in order to form the 1,4 glycosidic bonds consecutive β-glucose molecules must be rotated 180° to each other
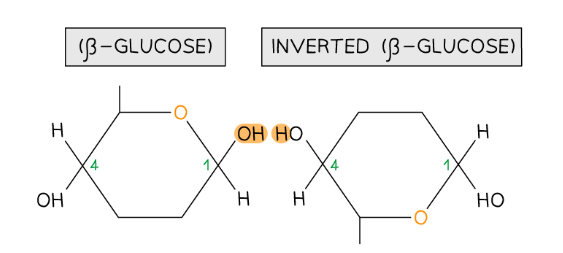
To form the 1,4 glycosidic bond between two β-glucose molecules, the glucose molecules must be rotated to 180° to each other
- Due to the inversion of the β-glucose molecules, many hydrogen bonds form between the long chains giving cellulose its strength
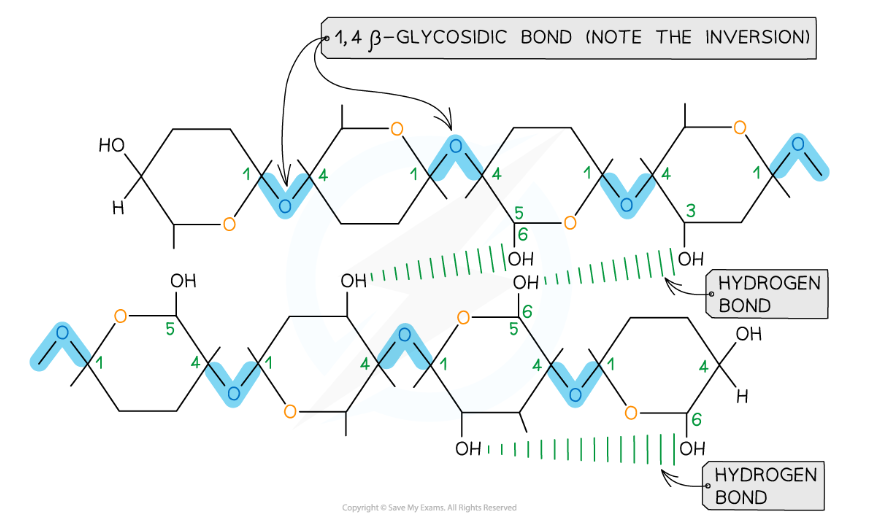
Cellulose has high tensile strength due to the many hydrogen bonds that form between the long chains of β-glucose molecules
Exam Tip
Be clear about the differences between starch, glycogen, and cellulose.
Polysaccharides: Function
- Starch and glycogen are storage polysaccharides because they are:
- Compact
- So large quantities can be stored
- Insoluble
- So they will have no osmotic effect, unlike glucose which would lower the water potential of a cell causing water to move into cells
- Compact
Starch
- Starch is the storage polysaccharide of plants. It is stored as granules in plastids such as amyloplasts and chloroplasts
- Plastids are membrane-bound organelles that can be found in plant cells. They have a specialised function eg. amyloplasts store starch grains
- Due to the many monomers in a starch molecule, it takes longer to digest than glucose
- The amylopectin in starch has branches that result in many terminal glucose molecules that can be easily hydrolysed for use during cellular respiration or added for storage
Glycogen
- Glycogen is the storage polysaccharide of animals and fungi, it is highly branched and not coiled
- Liver and muscles cells have a high concentration of glycogen, present as visible granules, as the cellular respiration rate is high in these cells (due to animals being mobile)
- Glycogen is more branched than amylopectin making it more compact which helps animals store more
- The branching enables more free ends where glucose molecules can either be added or removed allowing for condensation and hydrolysis reactions to occur more rapidly – thus the storage or release of glucose can suit the demands of the cell
Cellulose
- Cellulose is the main structural component of cell walls due to its strength which is a result of the many hydrogen bonds found between the parallel chains of microfibrils
- The high tensile strength of cellulose allows it to be stretched without breaking which makes it possible for cell walls to withstand turgor pressure
- The cellulose fibres and other molecules (eg. lignin) found in the cell wall forms a matrix which increases the strength of the cell walls
- The strengthened cell walls provide support to the plant
- Cellulose fibres are freely permeable which allows water and solutes to leave or reach the cell surface membrane
- As few organisms have the enzyme (cellulase) to hydrolyse cellulose it is a source of fibre
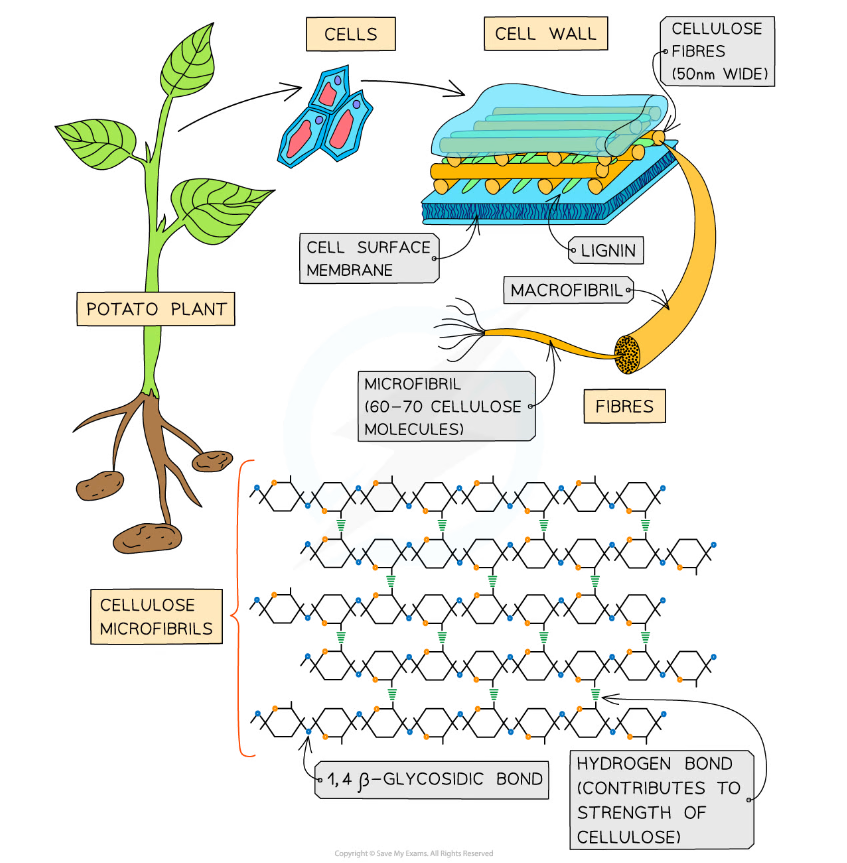
The strength and insolubility of cellulose fibres mean it is a suitable molecule to construct cell walls
Exam Tip
Other carbohydrate polymers exist in organisms: peptidoglycan is found in the cell walls of bacteria and chitin is found in the exoskeleton of insects.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





