- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:4.5.1 Addition Polymers
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:4.5.1 Addition Polymers
Monomers
- Polymers are large molecules of high relative molecular mass and are made by linking together large numbers of smaller molecules called monomers
- Each monomer is a repeat unit and is connected to the adjacent units via covalent bonds
- Polymerisation reactions usually require high pressures and the use of a catalyst
- Many everyday materials such as resins, plastics, polystyrene cups, nylon etc. are polymers
- These are manufactured and are called synthetic polymers
- Nature also produces polymers which are called natural or biological polymers
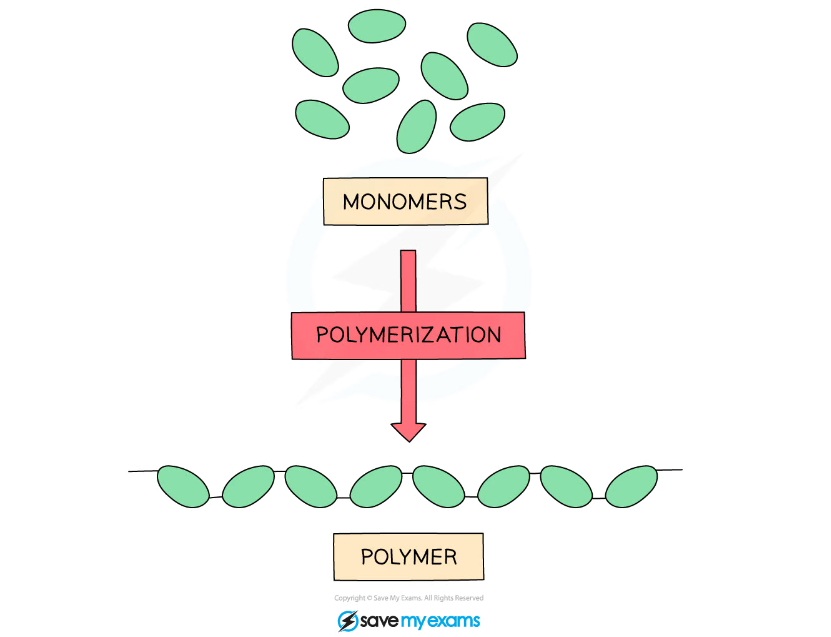
Diagram showing how lots of monomers bond together to form a polymer
Drawing Polymers
- Addition polymers are formed by the joining up of many monomers and only occurs in monomers that contain C=C bonds
- One of the bonds in each C=C bond breaks and forms a bond with the adjacent monomer with the polymer being formed containing single bonds only
- Many polymers can be made by the addition of alkene monomers
- Others are made from alkene monomers with different atoms attached to the monomer such as chlorine or a hydroxyl group
- The name of the polymer is deduced by putting the name of the monomer in brackets and adding poly- as the prefix
- For example if propene is the alkene monomer used, then the name is polypropene
- Polyethene is formed by the addition polymerisation of ethene monomers
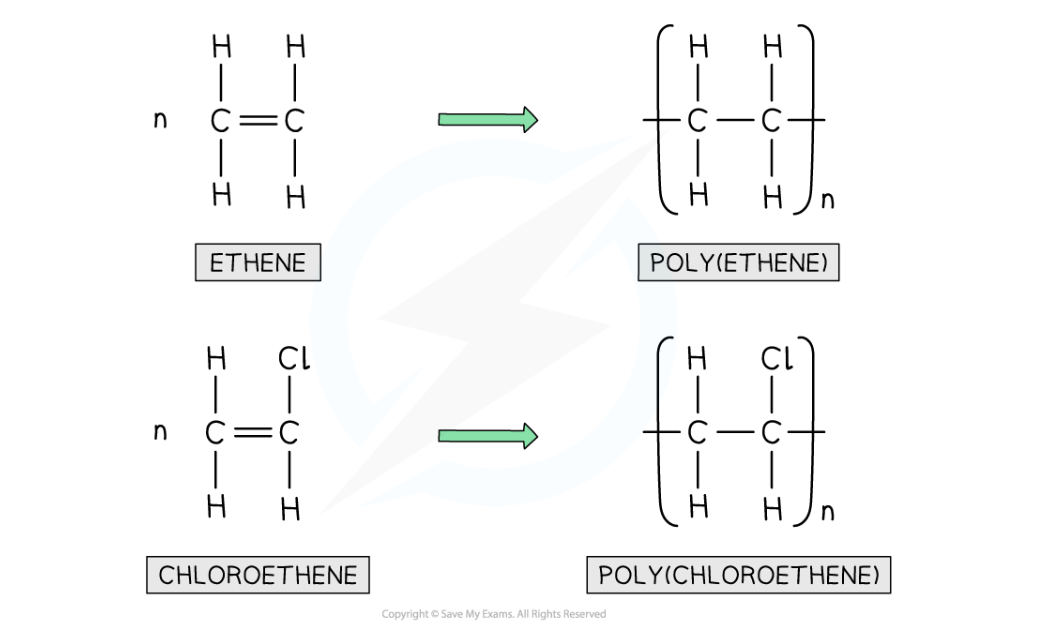
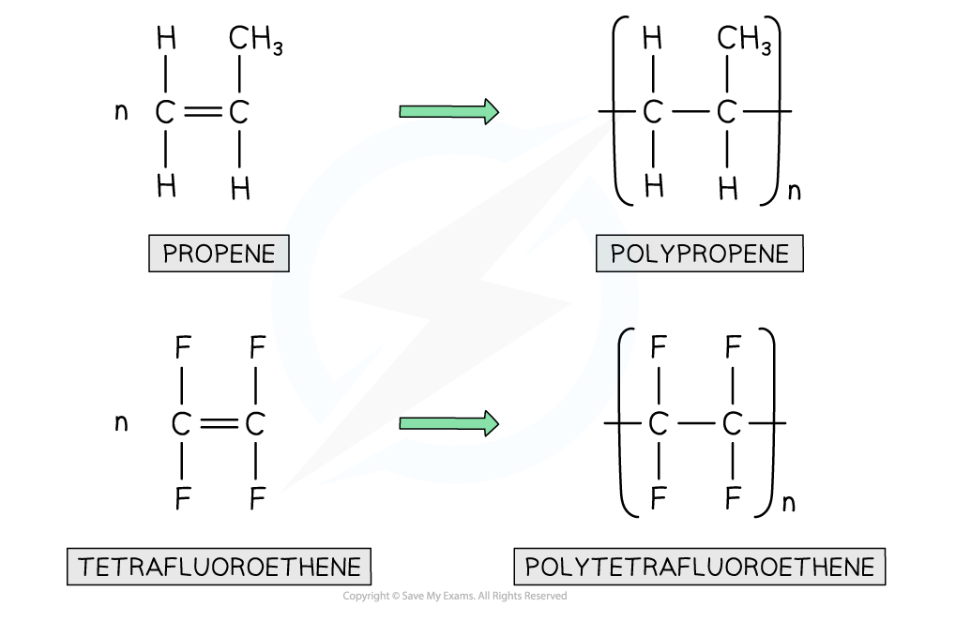
Examples of addition polymerisation: polyethene and PVC
Deducing Monomers & Repeat Units
Deducing the monomer from the polymer
- Polymer molecules are very large compared with most other molecule
- Repeat units are used when displaying the formula
- To draw a repeat unit, change the double bond in the monomer to a single bond in the repeat unit
- Add a bond to each end of the repeat unit
- The bonds on either side of the polymer must extend outside the brackets (these are called extension or continuation bonds)
- A small subscript n is written on the bottom right hand side to indicate a large number of repeat units
- Add on the rest of the groups in the same order that they surrounded the double bond in the monomer

Diagram showing the concept of drawing a repeat unit of a monomer
Deducing the polymer from the monomer
- Identify the repeating unit in the polymer
- Change the single bond in the repeat unit to a double bond in the monomer
- Remove the bond from each end of the repeat unit and the subscript n (which can be placed in front of the monomer)
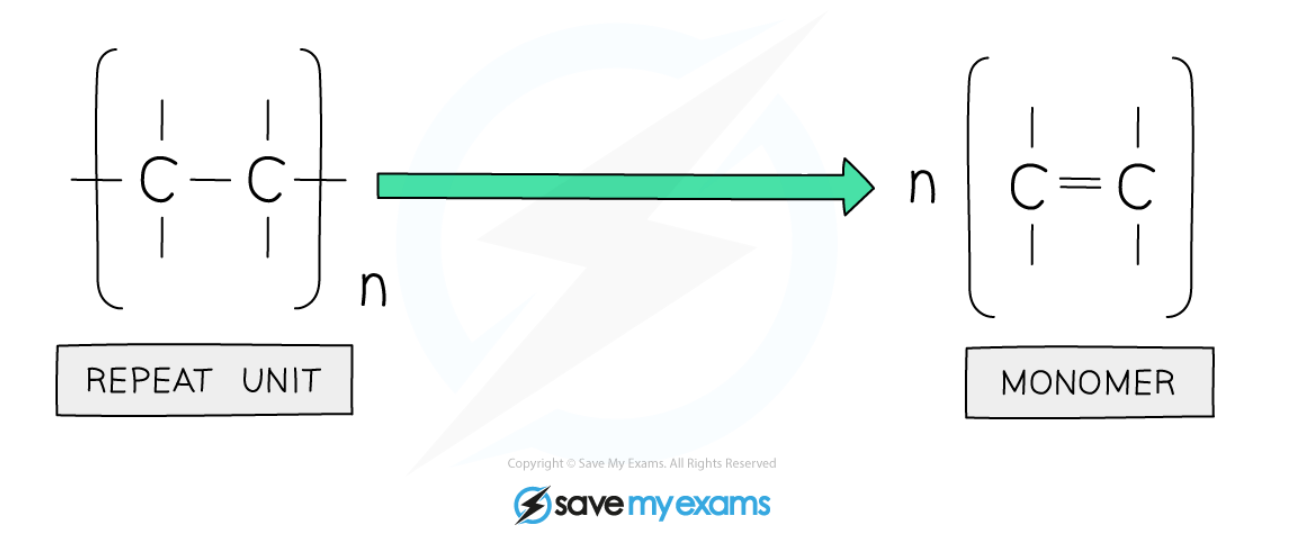
Diagram showing the monomer of the repeat unit of polymer
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





