- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:4.4.1 Alkenes
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:4.4.1 Alkenes
The Alkenes
- All alkenes contain a double carbon bond, which is shown as two lines between two of the carbon atoms i.e. C=C
- All alkenes contain a double carbon bond, which is the functional group and is what allows alkenes to react in ways that alkanes cannot
- The names and structure of the first four alkenes are shown below:
Table Showing the Formulae and Structures of the First Four Alkenes
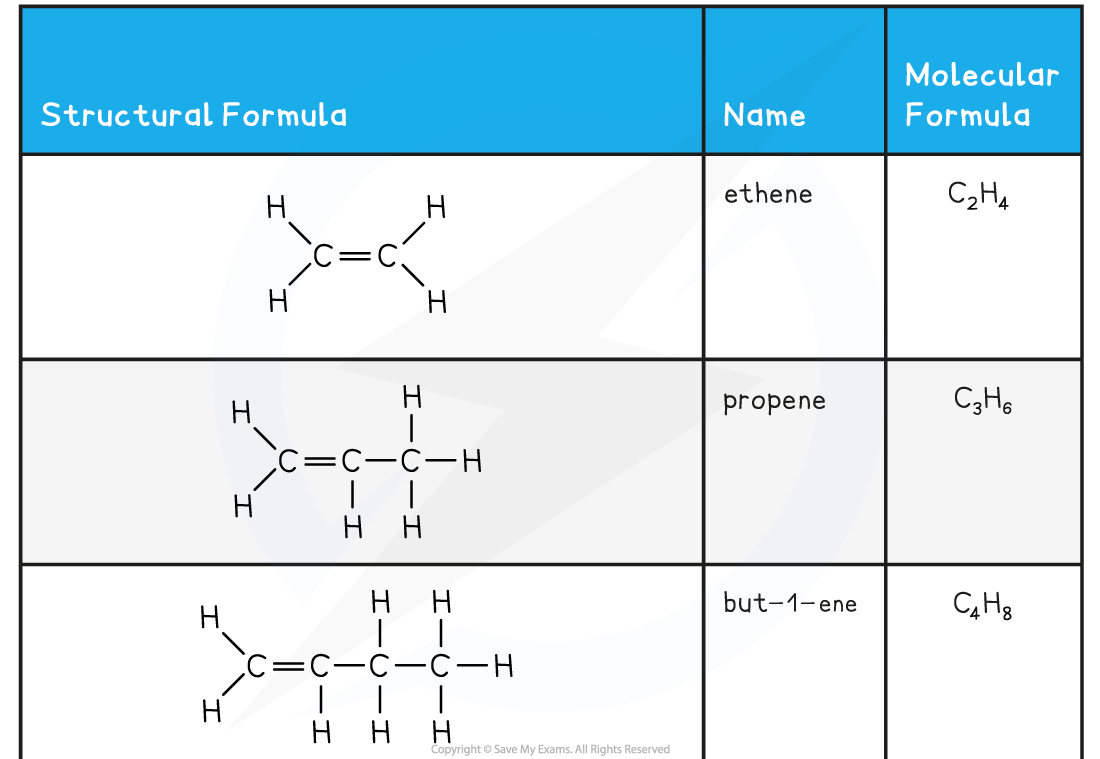
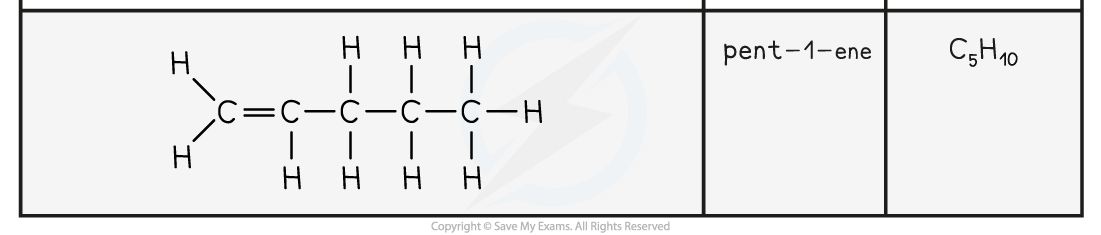
- Compounds that have a C=C double bond are also called unsaturated compounds
- That means they can make more bonds with other atoms by opening up the C=C bond and allowing incoming atoms to form another single bond with each carbon atom of the functional group
- Each of these carbon atoms now forms 4 single bonds instead of 1 double and 2 single bonds
- This makes them much more reactive than alkanes

A carbon-carbon double can break and form a single bond, allowing more atoms to attach to the carbon atoms
Exam Tip
The numbers in butene, pentene and hexene refer to the carbon atom in which the C=C begins, counting from the left. E.g. pent-2-ene, C5H10 has the C=C between the 2nd and 3rd carbon atoms. In pent-3-ene the C=C bond is between the 3rd and 4th carbon atoms from the left.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





