- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:4.2.3 Nitrogen Oxides & Sulfur Dioxide
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:4.2.3 Nitrogen Oxides & Sulfur Dioxide
Formation of Nitrogen Oxides
Nitrogen Oxides
- These compounds (NO and NO2) are formed when nitrogen and oxygen react in the high pressure and temperature conditions of internal combustion engines and blast furnaces
- Exhaust gases also contain unburned hydrocarbons and carbon monoxide
- Cars are fitted with catalytic converters which form a part of their exhaust systems
- Their function is to render these exhaust gases harmless
Adverse Effects
- Acid rain with similar effects as SO2 as well as producing photochemical smog and breathing difficulties, in particular for people suffering from asthma
Catalytic Converters
- They contain a series of transition metal catalysts including platinum and rhodium
- The metal catalysts are in a honeycomb within the converter to increase the surface area available for reaction
- A series of redox reactions occurs which neutralises the pollutant gases
- Carbon monoxide is oxidised to carbon dioxide:
2CO + O2 → 2CO2
- Nitrogen oxides are reduced to N2 gas:
2NO → N2 + O2
2NO2 → N2 + 2O2
- Unburned hydrocarbons are oxidised to carbon dioxide and water:
C8H18 + 12½O2 → 8CO2 + 9H2O
 Catalytic converters are designed to reduce the polluting gases produced in car exhausts
Catalytic converters are designed to reduce the polluting gases produced in car exhausts
Formation of Sulfur Dioxide
Sulfur dioxide
- Sources: combustion of fossil fuels - especially coal
- Fossil fuels are often contaminated with small amounts of sulfur impurities
- When these contaminated fossil fuels are combusted, the sulfur in the fuels get oxidised to sulfur dioxide
S (s) + O2 (g) → SO2 (g)
- Adverse effects: acid rain which causes corrosion to metal structures, buildings and statues made of carbonate rocks, damage to aquatic organisms. Pollutes crops and water supplies, irritates lungs, throats and eyes
Acid Rain
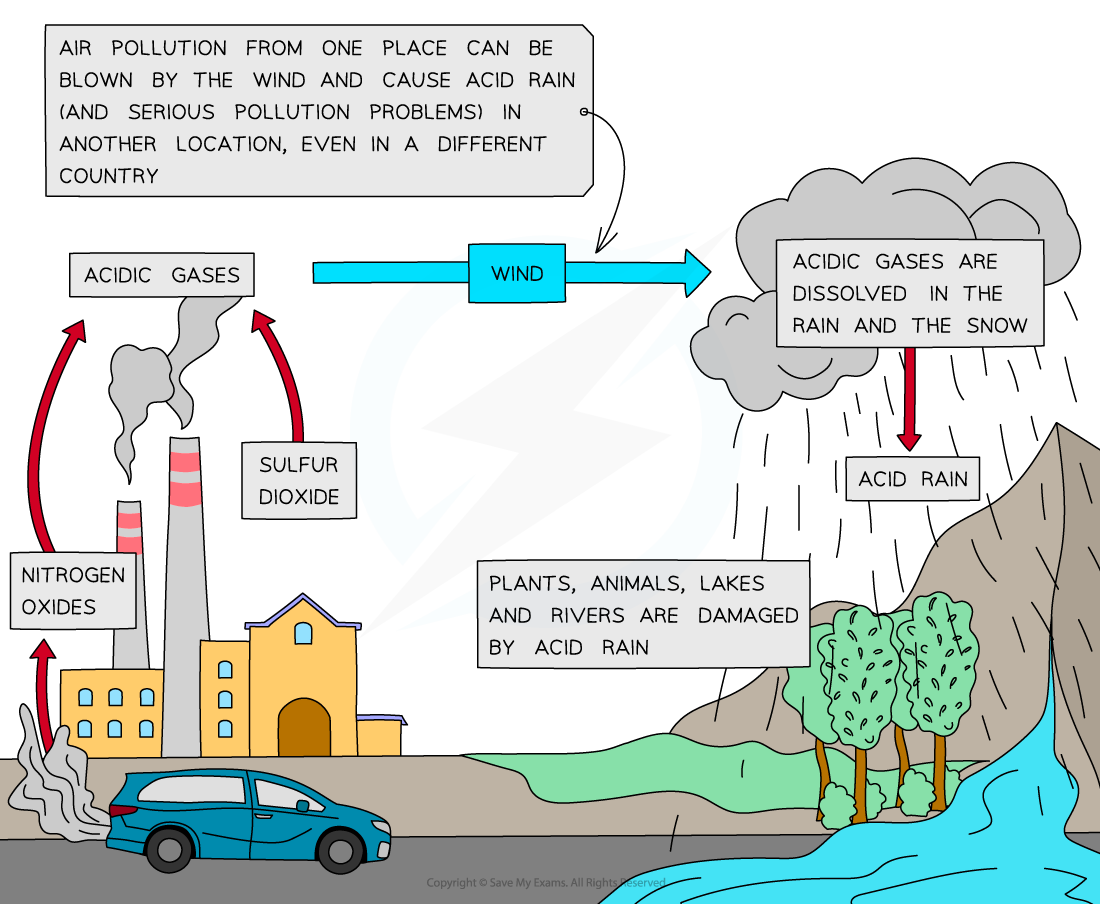
Formation of acid rain from nitrogen and sulfur oxides
- The sulfur dioxide produced from the combustion of fossil fuels dissolves in rainwater droplets to form sulfuric acid
2SO2 (g) + O2 (g) + 2H2O (l) → 2H2SO4 (aq)
- Sulfuric acid is one of the components of acid rain which has several damaging impacts on the environment
- Nitrogen dioxide produced from car engines reacts with rain water to form a mixture of nitrous and nitric acids, which contribute to acid rain:
2NO2 (g) + H2O (l) → HNO2 (aq) + HNO3 (aq)
- Lightning strikes can also trigger the formation of nitrogen monoxide and nitrogen dioxides in air
- Nitrogen dioxide gas reacts with rain water and more oxygen to form nitric acid
4NO2 (g) + 2H2O (l) + O2 (g)→ 4HNO3 (aq)
- When the clouds rise, the temperature decreases, and the droplets get larger
- When the droplets containing these acids are heavy enough, they will fall down as acid rain
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





