Practical: Investigate Metals Reacting with Acids
Aim:
To investigate the reactions between dilute hydrochloric and sulfuric acids with the metals magnesium, iron and zinc
Diagram:
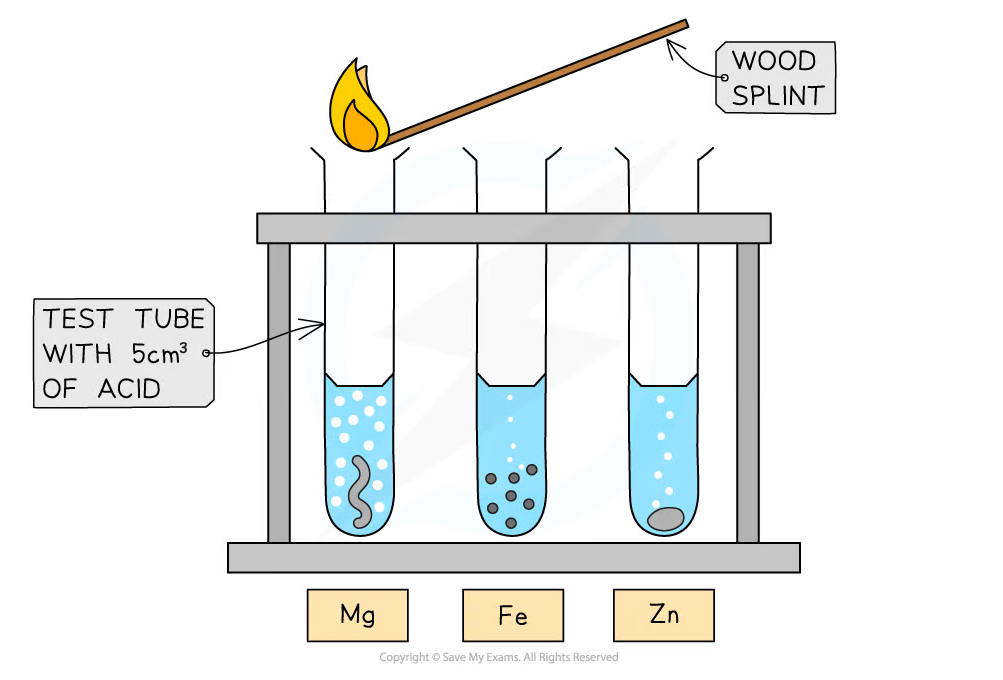
Investigating the reactions of dilute acids with metals
Method:
- Wear some safety glasses before handling acids
- Using a small measuring cylinder, add 5 cm3 of dilute hydrochloric acid to each of three test tubes
- Add about 1 cm length of magnesium ribbon to the first tube, observe and note down what you see
- Use a lighted splint to test for any gases given off
- To the second test tube add a few pieces of iron filings and to the third some zinc turnings
- Observe what happens, test for any gases and note down your observations
- Repeat the experiment with dilute sulfuric acid
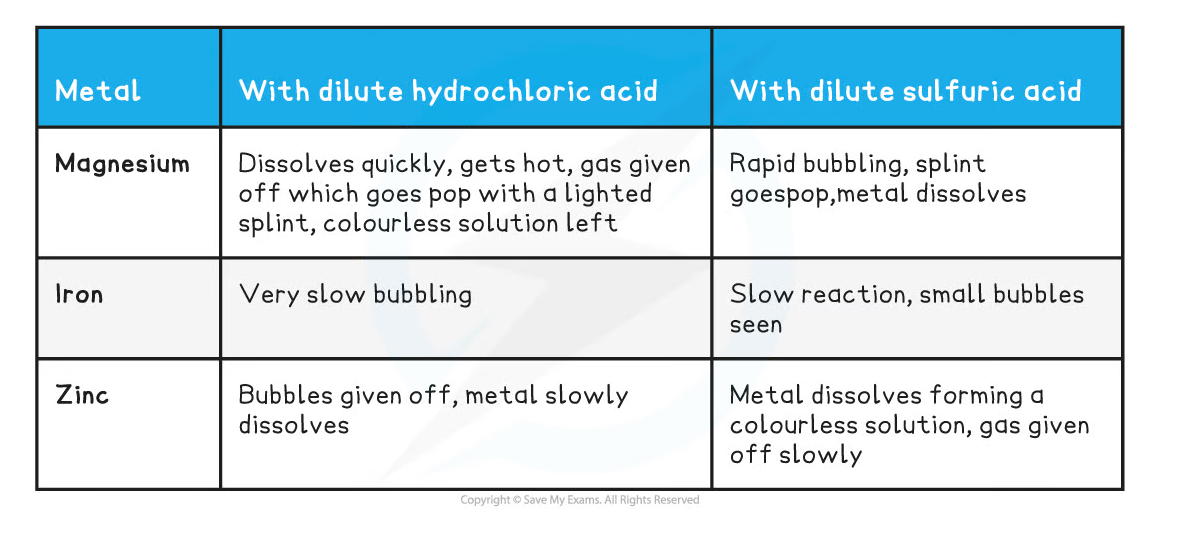
Results:
Metals with Acids Observations Table
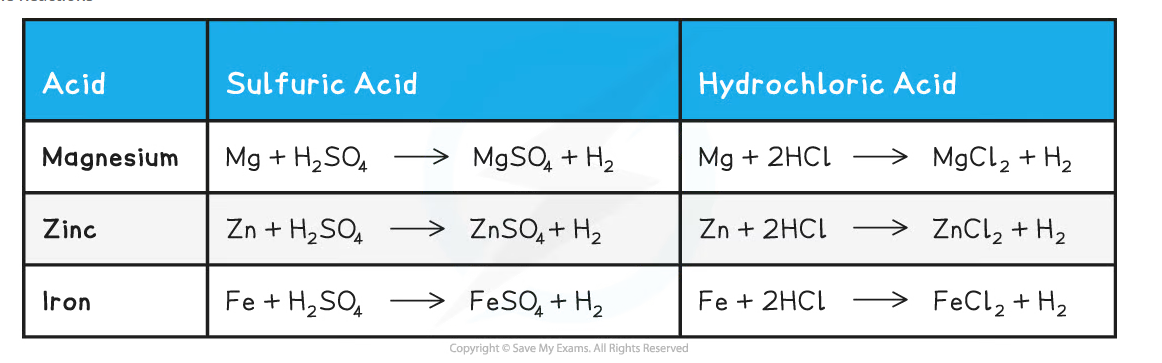
Equations for the Reactions
Conclusions:
- The metals can be ranked in reactivity order Mg > Zn > Fe
- The three metals react in the same with both acids
- Hydrogen and a metal salt solution is produced
转载自savemyexams





