- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:2.3.4 Carbon Dioxide from Thermal Decomposition
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:2.3.4 Carbon Dioxide from Thermal Decomposition
Carbon Dioxide from Thermal Decomposition
- Thermal decomposition is the term used to describe reactions where a substance breaks down due to the action of heat
- One such reaction is the thermal decomposition of metal carbonates
- Carbonates of metals from the lower half of the reactivity series tend to decompose on heating to produce the metal oxide and carbon dioxide gas:
metal carbonate → metal oxide + carbon dioxide
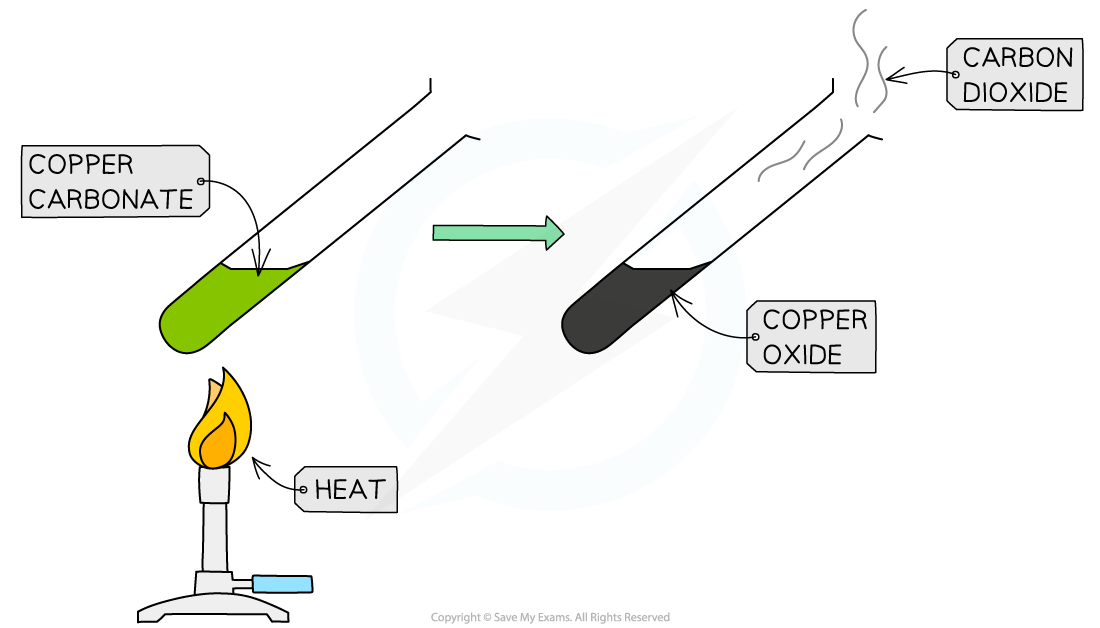
The thermal decomposition of copper(II)carbonate
- The thermal decomposition of copper(II)carbonate occurs readily on heating
- Copper(II) carbonate is a green powder and slowly darkens as black copper(II) oxide is produced
- The carbon dioxide given off can be tested by passing the gas through limewater and looking for it to turn milky
- The equation for the reaction is
CuCO3 (s) → CuO (s)+ CO2 (g)
copper(II) carbonate → copper(II) oxide + carbon dioxide
Exam Tip
The release of carbon dioxide from calcium carbonate in the production of cement is a contributing source of rising atmospheric CO2 levels that contributes to the enhanced greenhouse effect.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





