- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.6.2 Common Ions
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.6.2 Common Ions
Common Ions
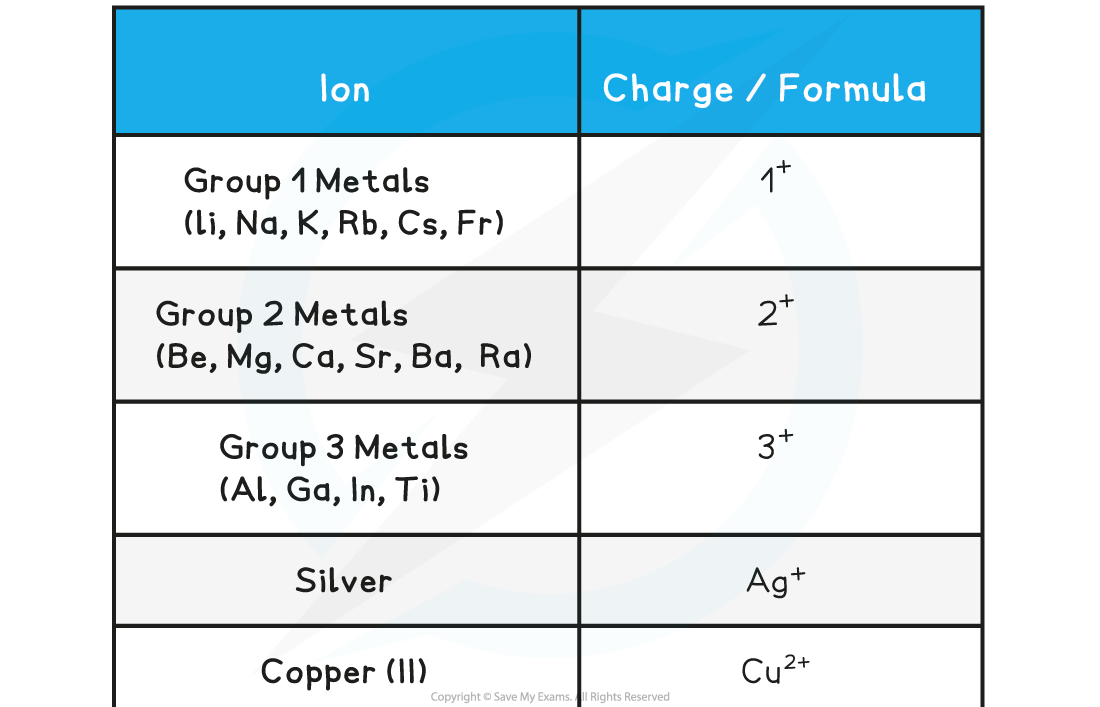
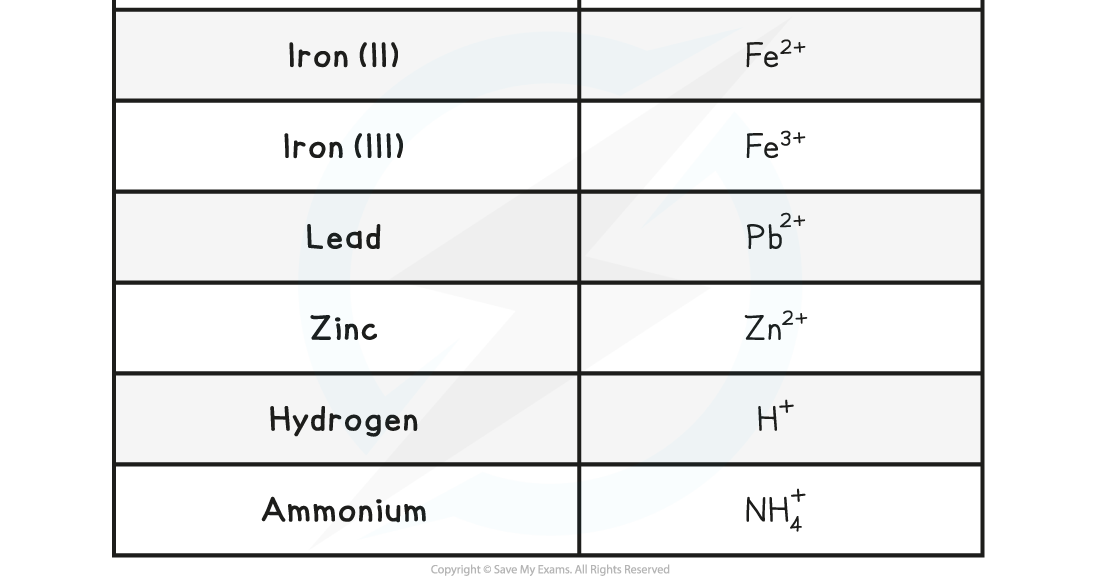
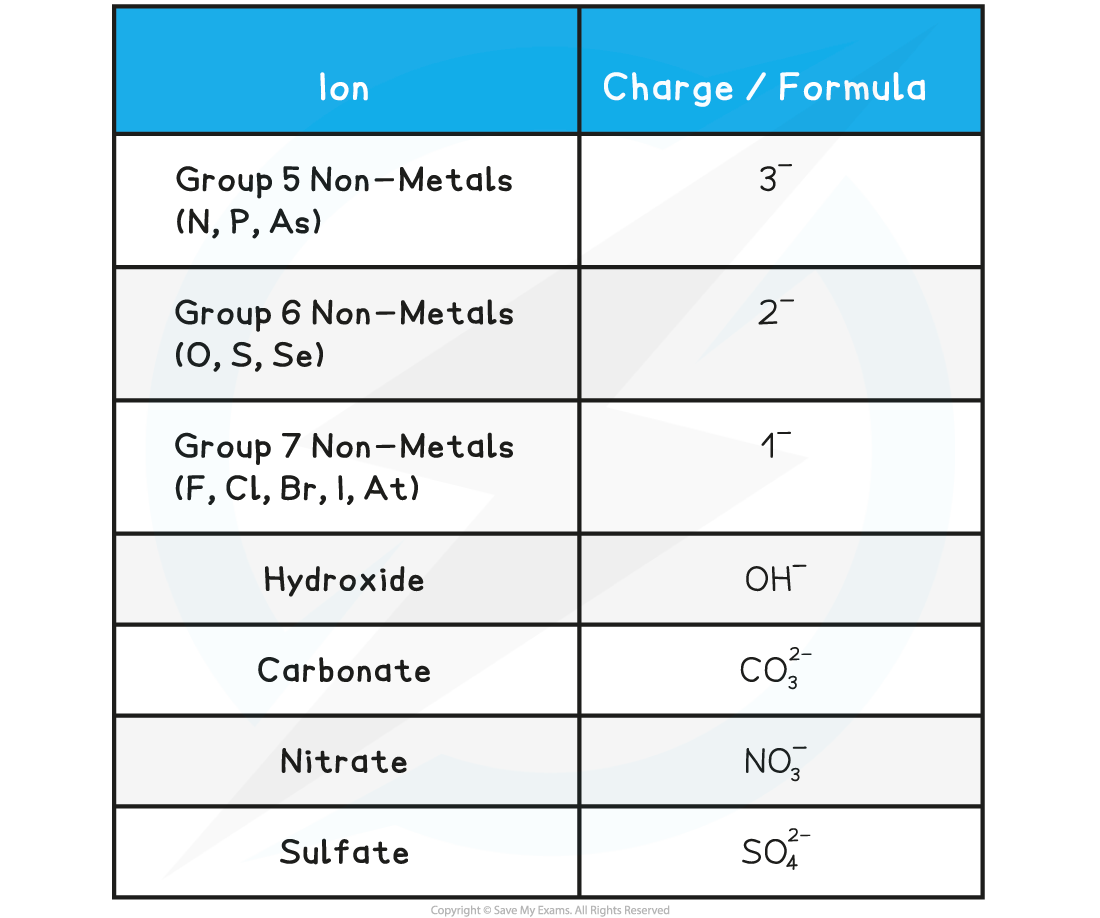
How to deduce the charge of an ion
- Find the number of electrons in the outer electron shell
- Find out if it is easy for the atom to gain electron or to donate electron (in most cases atoms that have fewer than four electrons, donate electrons and atoms that have more than 4 electrons, receive electrons)
- Atoms that gain electrons become negative ions and atoms that donate electron forms positive ion
- You also need to learn the formula of compound ions, that is, ions made from more than one element
The Charges of Common Positive Ions Table
The Charges of Common Negative Ions Table
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





