- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.5.7 Practical: Determine the Formula of a Metal Oxide
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.5.7 Practical: Determine the Formula of a Metal Oxide
Practical: Determine the Formula of Magnesium Oxide
Aim:
To determine the empirical formula of magnesium oxide by combustion of magnesium
Diagram:
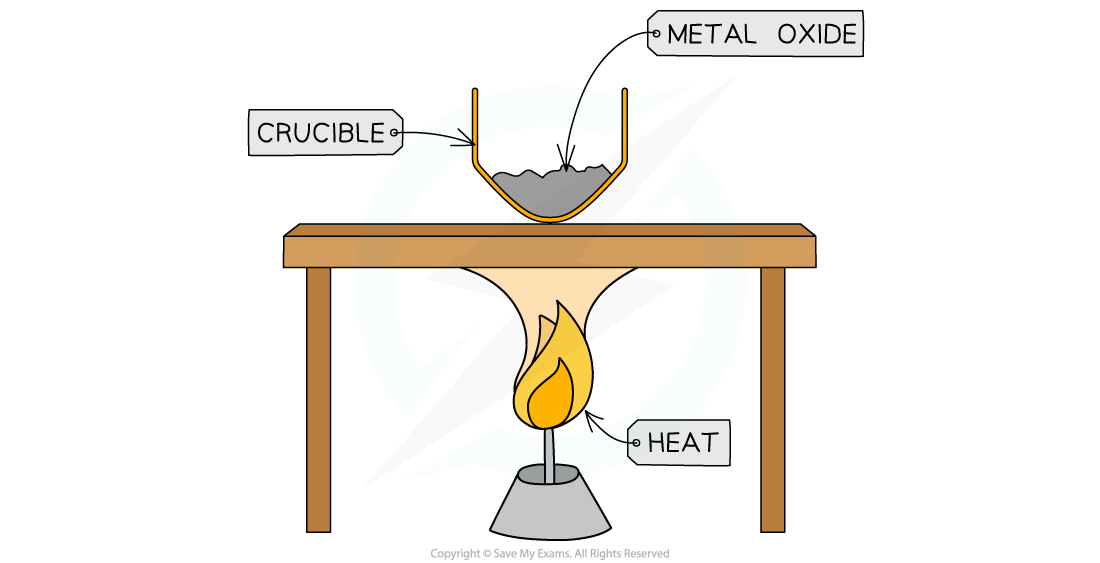
Finding the empirical formula of magnesium oxide
Method:
- Measure mass of crucible with lid
- Add sample of magnesium into crucible and measure mass with lid (calculate the mass of the metal by subtracting the mass of empty crucible)
- Strongly heat the crucible over a Bunsen burner for several minutes
- Lift the lid frequently to allow sufficient air into the crucible for the magnesium to fully oxidise without letting magnesium oxide smoke escape
- Continue heating until the mass of crucible remains constant (maximum mass), indicating that the reaction is complete
- Measure the mass of crucible and contents (calculate the mass of metal oxide by subtracting the mass of empty crucible)
Working out the empirical formula:
Mass of metal:
Subtract mass of crucible from magnesium and the mass of the empty crucibleMass of oxygen:Subtract mass of the magnesium used from the mass of magnesium oxide
Step 1 – Divide each of the two masses by the relative atomic masses of the elements
Step 2 – Simplify the ratio
magnesium oxygen
Mass a b
Mole a / Ar b / Ar
= x = y
Ratio x : y
Step 3 – Represent the ratio into the form ‘MxOy‘ E.g, MgO
Practical: Determine the Formula of Copper(II)Oxide
Aim:
To determine the formula of copper(II)oxide by reduction with methane
Diagram:
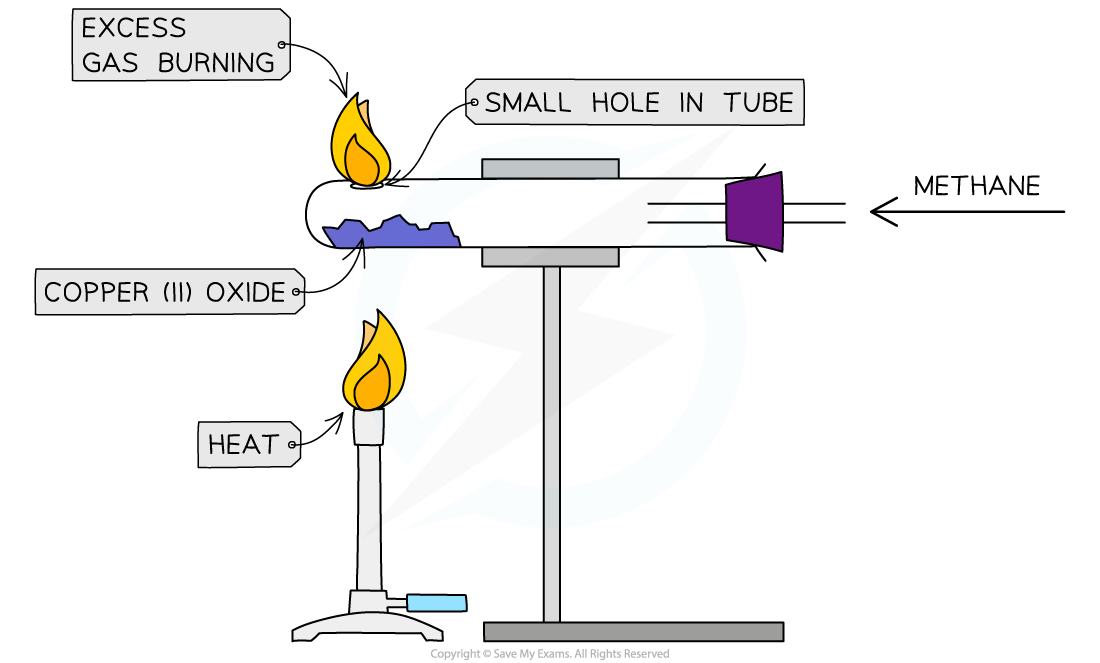
Finding the empirical formula of copper(II)oxide
Method:
- Measure mass of the empty boiling tube
- Place metal oxide into a horizontal boiling tube and measure the mass again
- Support the tube in a horizontal position held by a clamp
- A steady stream of natural gas(methane) is passed over the copper(II)oxide and the excess gas is burned off
- The copper(II)oxide is heated strongly using a Bunsen burner
- Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed
- Measure mass of the tube remaining metal powder and subtract the mass of the tube
Working out empirical formula:Mass of Metal:Measure mass of the remaining metal powder
Mass of Oxygen:
Subtract mass of the remaining metal powder from the mass of metal oxideStep 1 – Divide each of the two masses by the relative atomic masses of elementsStep 2 – Simplify the ratio Metal OxygenMass a bMole a / Mr b / Mr= x = yRatio x : yStep 3 – Represent the ratio into the form ‘MxOy‘ E.g, CuO
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





