- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.5.5 Calculate Percentage Yield
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.5.5 Calculate Percentage Yield
Calculate Percentage Yield
- Yield is the term used to describe the amount of product you get from a reaction
- In practice, you never get 100% yield in a chemical process for several reasons
- These include:
- Some reactants may be left behind in the equipment
- The reaction may be reversible and in these reactions a high yield is never possible as the products are continually turning back into the reactants
- Some products may also be lost during separation and purification stages such as filtration or distillation
- There may be side reactions occurring where a substance reacts with a gas in the air or an impurity in one of the reactants
- Products can also be lost during transfer from one container to another
Actual & Theoretical Yield
- The actual yield is the recorded amount of product obtained
- The theoretical yield is the amount of product that would be obtained under perfect practical and chemical conditions
- It is calculated from the balanced equation and the reacting masses
- The percentage yield compares the actual yield to the theoretical yield
- For economic reasons, the objective of every chemical producing company is to have as high a percentage yield as possible to increase profits and reduce costs and waste
Percentage Yield
- The percentage yield is a good way of measuring how successful a chemical process is
- There are often several methods of creating a compound and each method is called a reaction pathway
- Reaction pathways consist of a sequence of reactions which must occur to produce the required product
- Companies often investigate and try out different reaction pathways and these are then compared and evaluated so that a manufacturing process can be chosen
- The percentage yield of each pathway is a significant factor in this decision making process
- The equation to calculate the percentage yield is:
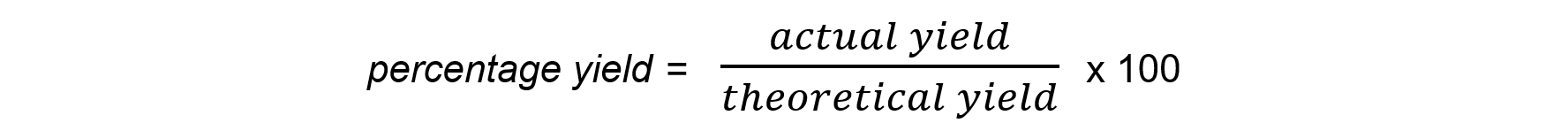
Worked Example
Copper(II) sulfate may be prepared by the reaction of dilute sulfuric acid on copper(II) oxide. A student prepared 1.6 g of dry copper(II) sulfate crystals. Calculate the percentage yield if the theoretical yield is 2.0 g.
Answer
-
- Actual yield of copper(II) sulfate = 1.6 g
- Percentage yield of copper(II) sulfate = (1.6 / 2.0) x 100
- Percentage yield = 80%
Exam Tip
The actual yield can be determined by experiment only, while the theoretical yield can be calculated assuming there is 100% conversion of reactants to products.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





