- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.5.2 Calculate Relative Mass
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.5.2 Calculate Relative Mass
Relative Formula Mass & Relative Molecular Mass
- We have seen previously that the symbol for the relative atomic mass is Ar
- This is calculated from the mass number and relative abundances of all the isotopes of a particular element
- The symbol for the relative formula mass is Mr and it refers to the total mass of the substance
- If the substance is molecular you can use the term relative molecular mass, but this term should not be used for ionic compounds such as sodium chloride
- To calculate the Mr of a substance, you have to add up the relative atomic masses of all the atoms present in the formula
Relative Formula Mass Calculations Table
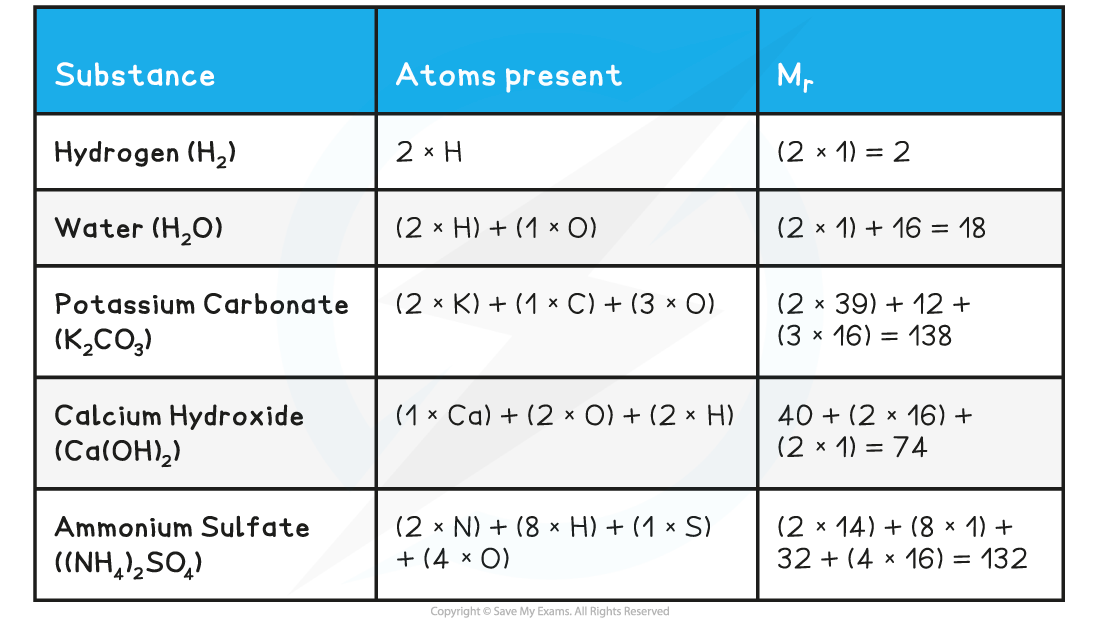
- In accordance with the Law of Conservation of Mass, the sum of the relative formula masses of the reactants will be the same as the sum of the relative formula masses of the products
Exam Tip
If you are in any doubt whether to use relative molecular mass or relative formula mass, use the latter because it applies to all compounds whether they are ionic or covalent.
Calculating percentage by mass of an element in a compound
- The percentage by mass of an element in a compound can be calculated using the following equation:
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





