- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.3.1 Atoms: Definitions & Structure
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.3.1 Atoms: Definitions & Structure
Atoms & Molecules
- It is important to understand the terms atom and molecule when referring to atomic structure, elements and compounds
Atoms & Molecules Definitions Table

The Structure of the Atom
- Elements are made of tiny particles of matter called atoms
- Each atom is made of subatomic particles called protons, neutrons and electrons
- Their size is so tiny that we can't really compare their masses in conventional units such as kilograms or grams, so a unit called the relative atomic mass is used
- One relative atomic mass unit is equal to one twelfth the mass of a carbon-12 atom.
- All other elements are measured relative to the mass of a carbon-12 atom and since these are ratios, the relative atomic mass has no units
- Hydrogen for example has a relative atomic mass of 1, meaning that 12 atoms of hydrogen would have exactly the same mass as 1 atom of carbon
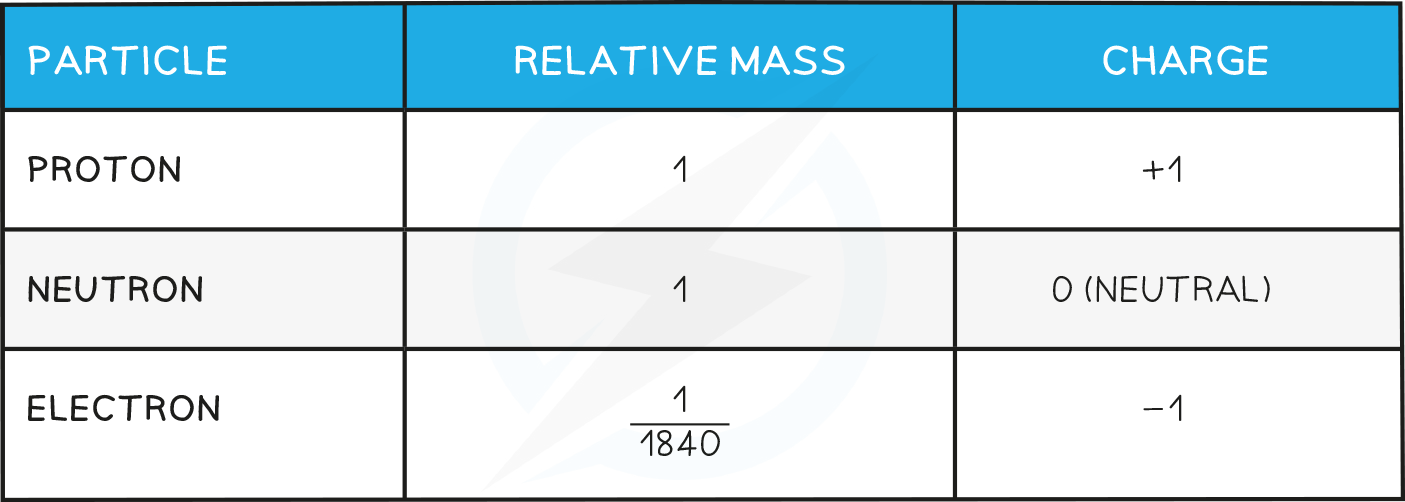
- The relative mass and charge of the subatomic particles are shown below:
The Mass & Charge of Subatomic Particles Table
Exam Tip
The mass of an electron can just be stated as 'negligible' or 'very small' in an exam. You do not need to learn the value.
Key Terms
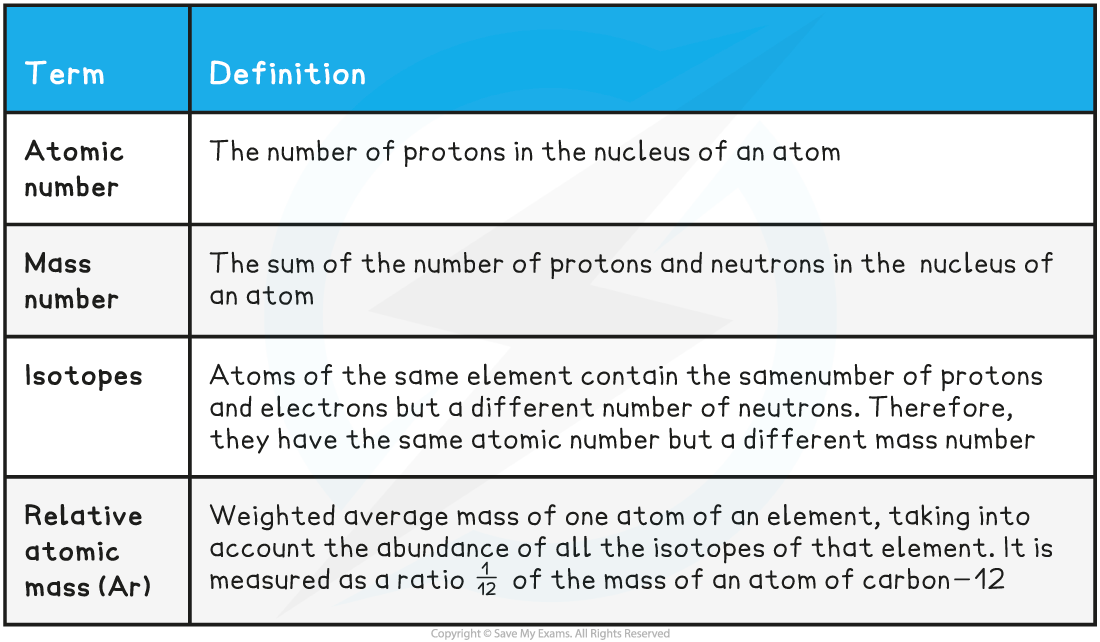
- You need to know the following terms to describe the properties and characteristics of atoms
Atomic Structure Key Terms Table
Exam Tip
The term nucleon number is an alternative to mass number and means the same thing. A nucleon is a collective name for protons and neutrons.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





