- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.2.4 Interpreting Chromatograms
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.2.4 Interpreting Chromatograms
Identifying Mixtures
- Pure substances will produce only one spot on the chromatogram
- If two or more substances are the same, they will produce identical chromatograms
- If the substance is a mixture, it will separate on the paper to show all the different components as separate spots
- An impure substance therefore will produce a chromatogram with more than one spot
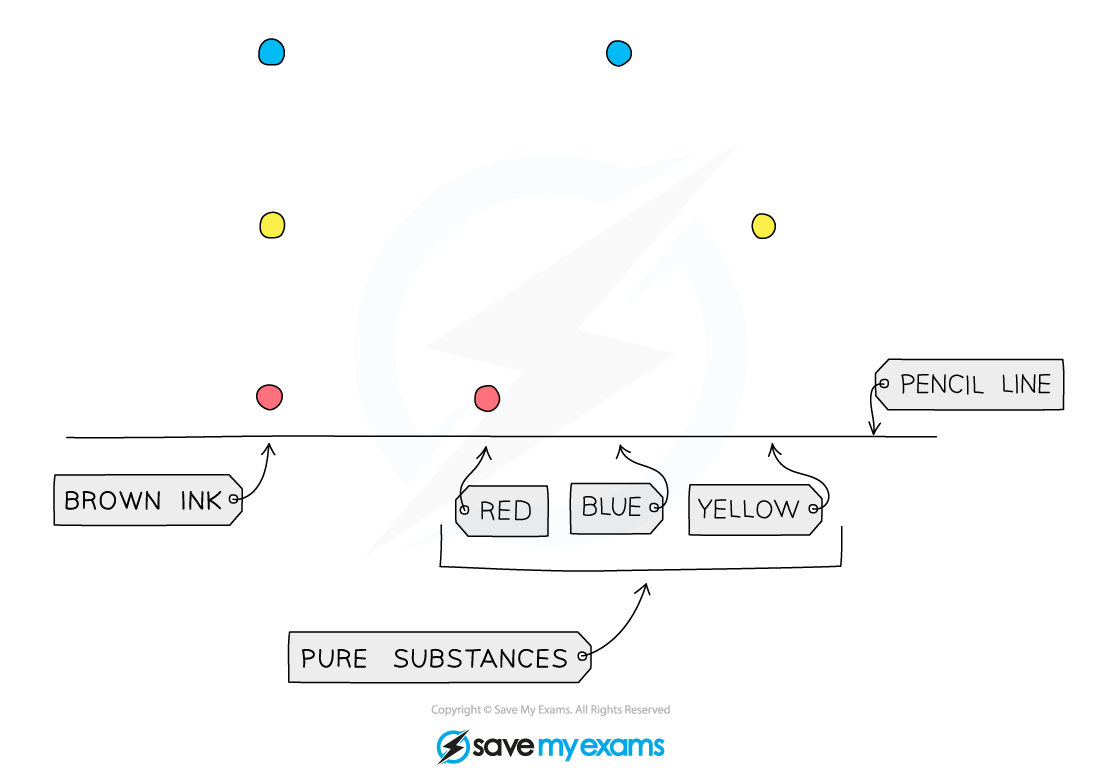
Diagram showing the analysis of a mixture and pure substances using chromatography
Rf Values
- These values are used to identify the components of mixtures
- The Rf value of a particular compound is always the same but it is dependent, however, on the solvent used
- If the solvent is changed then the value changes
- Calculating the Rf value allows chemists to identify unknown substances because it can be compared with Rf values of known substances under the same conditions
- These values are known as reference values
Calculation
- The Retention factor is found using the following calculation:
Rf = distance travelled by substance ÷ distance travelled by solvent
- The Rf value will always lie between 0 and 1; the closer it is to 1, the more soluble is that component in the solvent
- The Rf value is a ratio and therefore has no units
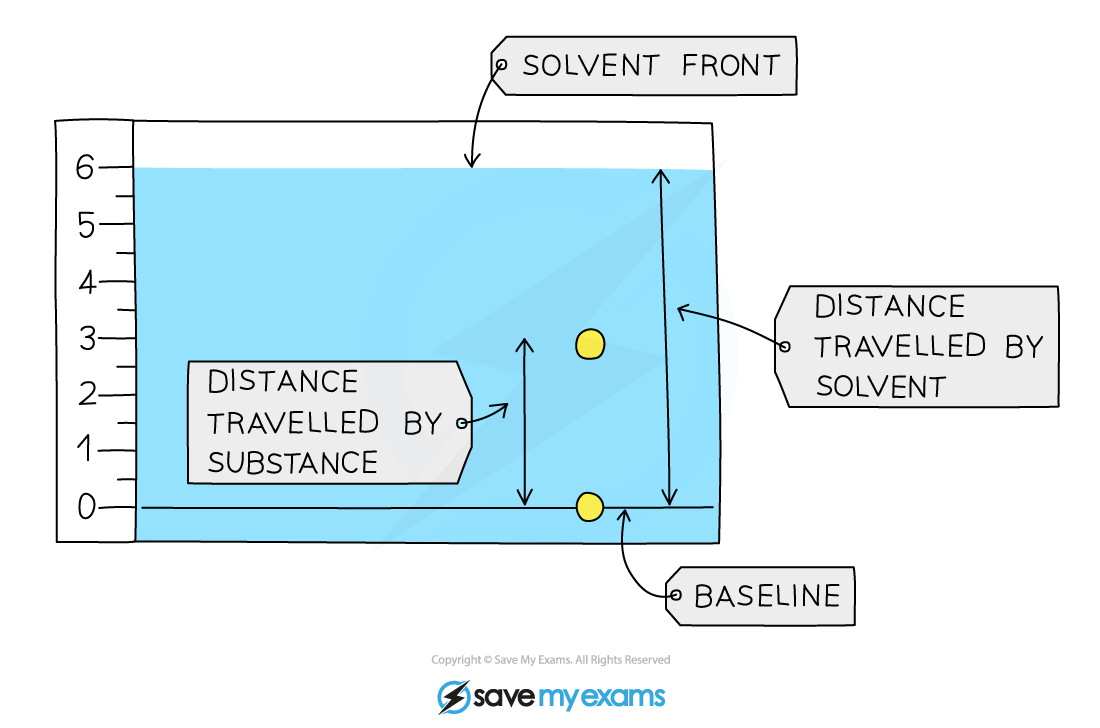
Using Rf values to identify components of a mixture
Exam Tip
For the Rf calculations, both distances are measured from the baseline.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





