- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry: Double Science 复习笔记:1.1.2 Diffusion & Dilution
Edexcel IGCSE Chemistry: Double Science 复习笔记:1.1.2 Diffusion & Dilution
iffusion & Dilution
- Diffusion and dilution experiments support a theory that all matter (solids, liquids and gases) is made up of tiny, moving particles
Diffusion in gases
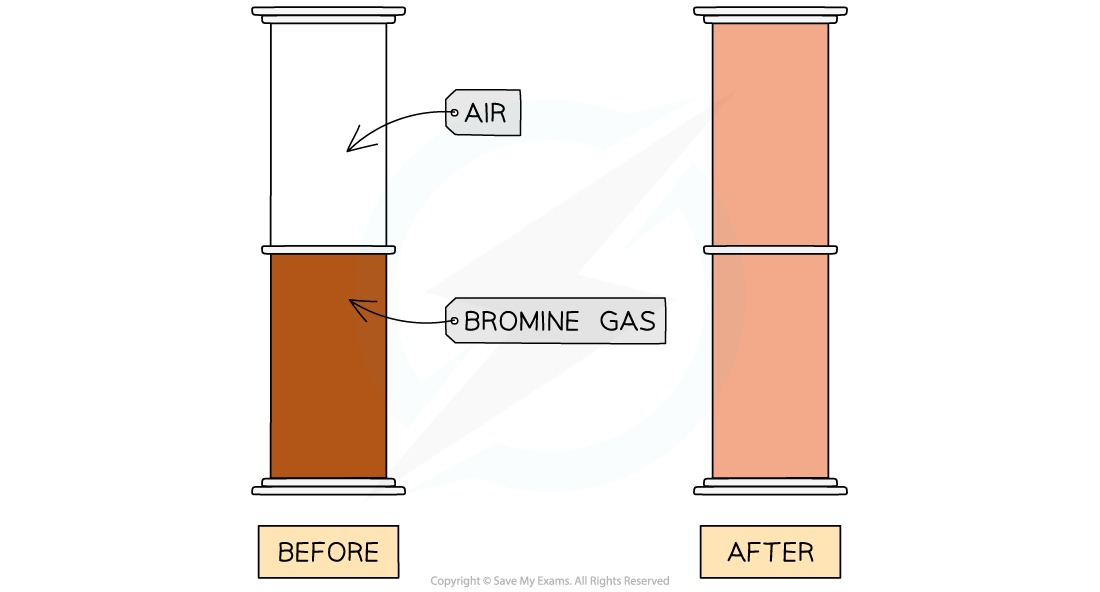
Diffusion of red-brown bromine gas
Description:
- Here, we see the diffusion of bromine gas from one gas jar to another
- After 5 minutes the bromine gas has diffused from the bottom jar to the top jar
Explanation:
- The air and bromine particles are moving randomly and there are large gaps between particles
- The particles can therefore easily mix together
Diffusion in liquids
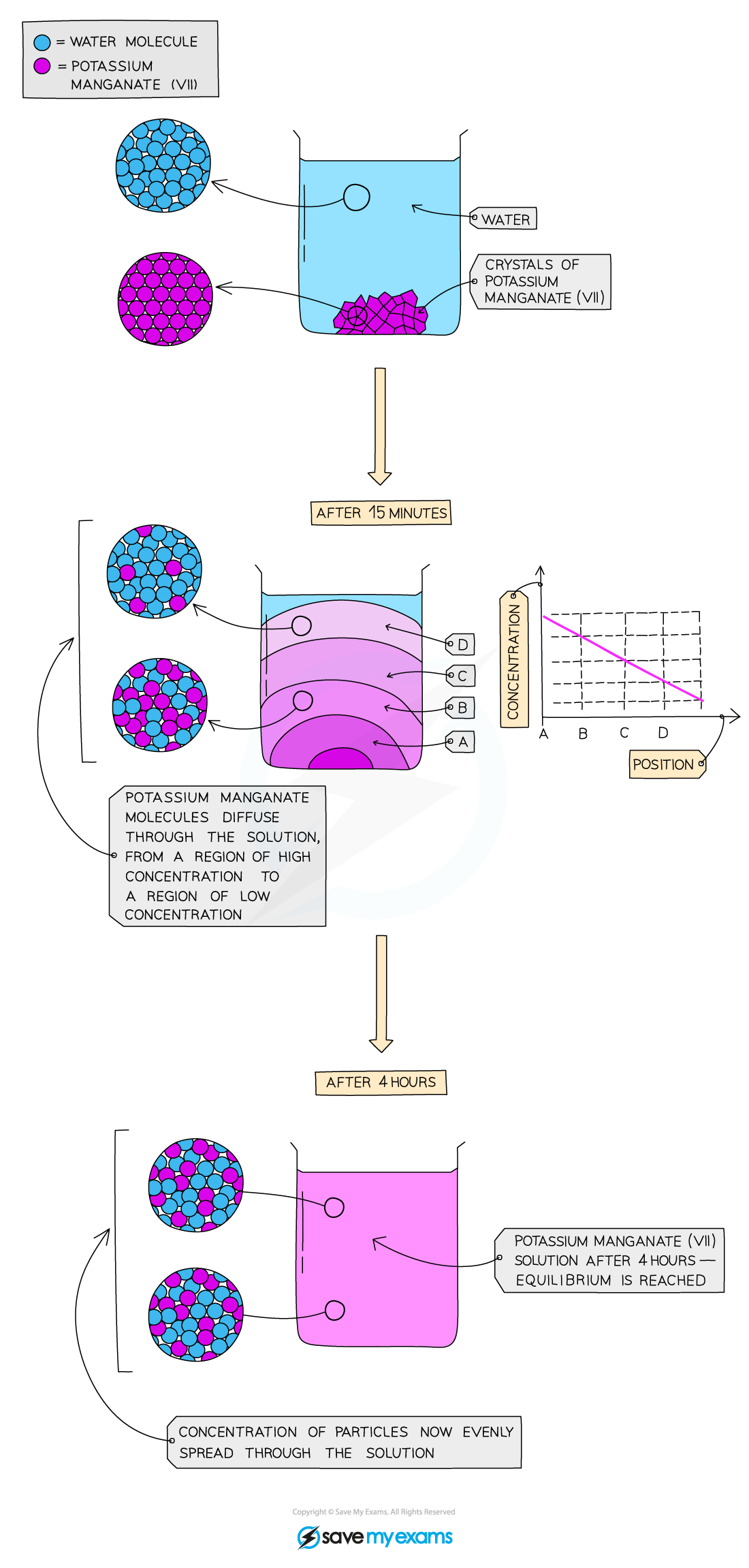
Diffusion of potassium manganate(VII) in water over time
Description:
- When potassium manganate (VII) crystals are dissolved in water, a purple solution is formed
- A small number of crystals produce a highly intense colour
Explanation:
- The water and potassium manganate (VII) particles are moving randomly and the particles can slide over each other
- The particles can therefore easily mix together
- Diffusion in liquids is slower than in gases because the particles in a liquid are closely packed together and move more slowly
Dilution
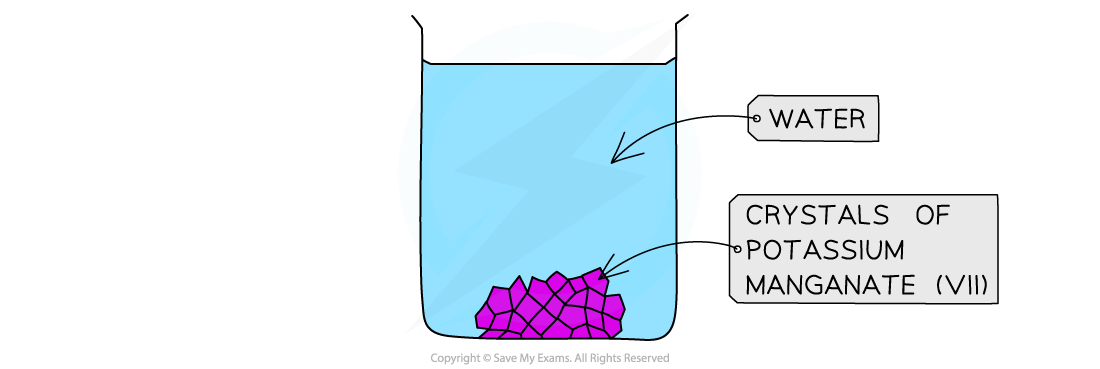
Dissolving potassium manganate (VII) in water
Description:
- When potassium magnate (VII) crystals are dissolved in water, the solution can be diluted several times
- The colour fades but does not disappear until a lot of dilutions have been done
Explanation:
- This indicates that there are a lot of particles in a small amount of potassium manganate (VII) and therefore the particles must be very small
Exam Tip
Diffusion and dilution provide evidence for the kinetic theory of matter.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





