- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A (SNAB) A Level Biology:复习笔记2.3.4 Amino Acids & Peptide Bonds
Amino Acid: Structure
Proteins
- Proteins are polymers (and macromolecules) made of monomers called amino acids
- The sequence, type and number of the amino acids within a protein determines its shape and therefore its function
- Proteins are extremely important in cells because they form all of the following:
- Enzymes
- Cell membrane proteins (eg. carrier)
- Hormones
- Immunoproteins (eg. immunoglobulins)
- Transport proteins (eg. haemoglobin)
- Structural proteins (eg. keratin, collagen)
- Contractile proteins (eg. myosin)
Amino acids
- Amino acids are the monomers of polypeptides
- There are 20 amino acids found in proteins common to all living organisms
- The general structure of all amino acids is a central carbon atom bonded to:
- An amine (also called amino) group -NH2
- A carboxylic acid group -COOH
- A hydrogen atom
- An R group (which is how each amino acid differs and why amino acid properties differ e.g. whether they are acidic or basic or whether they are polar or non-polar)
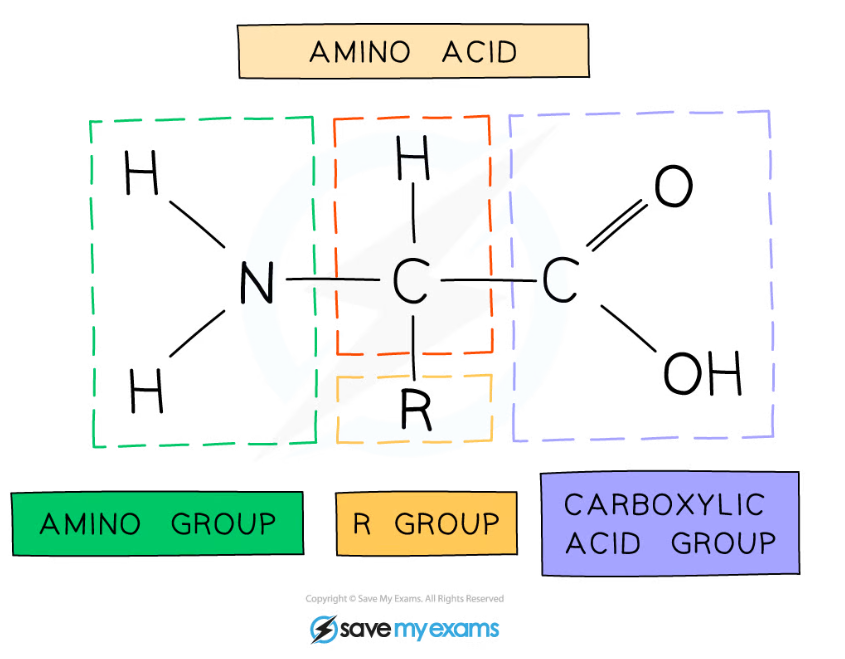
The general structure of an amino acid
The Peptide Bond
- Peptide bonds form between amino acids
- Peptide bonds are covalent bonds and so involve the sharing of electrons
- In order to form a peptide bond :
- A hydroxyl (-OH) is lost from the carboxylic group of one amino acid
- A hydrogen atom is lost from the amine group of another amino acid
- The remaining carbon atom (with the double-bonded oxygen) from the first amino acid bonds to the nitrogen atom of the second amino acid
- This is a condensation reaction so water is released
- Dipeptides are formed by the condensation of two amino acids
- Polypeptides are formed by the condensation of many (3 or more) amino acids
- A protein may have only one polypeptide chain or it may have multiple chains interacting with each other
- During hydrolysis reactions, the addition of water breaks the peptide bonds resulting in polypeptides being broken down to amino acids
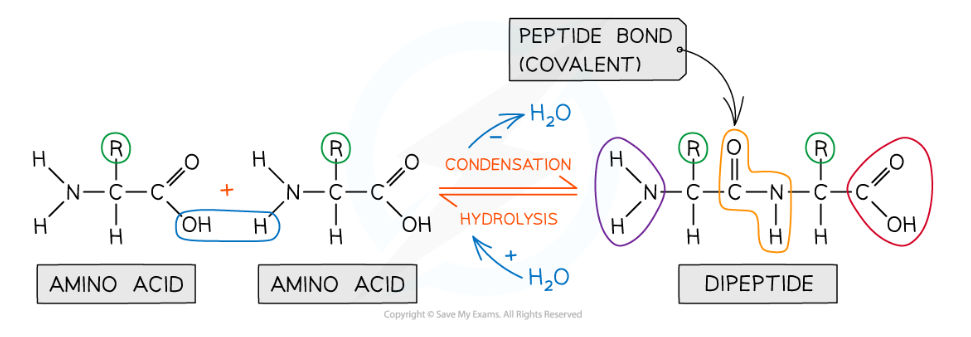
Peptide bonds are formed by condensation reactions (releasing a molecule of water) and broken by hydrolysis reactions (adding a molecule of water)
Exam Tip
When asked to identify the location of the peptide bond, look for where nitrogen is bonded to a carbon which has a double bond with an oxygen atom, note the R group is not involved in the formation of a peptide bond.
Structures of specific amino acids are not required.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





