- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A (SNAB) A Level Biology:复习笔记1.2.11 Practical: Vitamin C Content
1.2.11 Practical: Vitamin C Content
- Vitamin C is found in green vegetables, fruits, and potatoes
- It is essential for a healthy diet
- The chemical name for vitamin C is ascorbic acid
- Ascorbic acid is a good reducing agent and therefore it is easily oxidised
- Methods for the detection of vitamin C involve titrating it against a solution of an oxidising agent called DCPIP
- DCPIP is a blue dye that turns colourless in the presence of vitamin C
- Note that titration is a method of chemical analysis that involves determining the quantity of a substance present by gradually adding another substance
Apparatus
- Vitamin C solutions
- 1% DCPIP solution
- Distilled water
- Range of fruit juices
- Measuring cylinder
- Pipette
- Stop watch
- Test tubes
Method
- Make up a series. e.g. six, of known vitamin C concentrations
- This can be done by serial dilution
- Use a measuring cylinder to measure out 1 cm3 of DCPIP solution into a test tube
- Add one of the vitamin C solutions, drop by drop, to the DCPIP solution using a graduated pipette or burette
- Shake the tube for a set period of time using a stop watch
- It is important to keep the shaking time the same for each concentration; this is a control variable
- When the solution turns colourless record the volume, in number of drops, of vitamin C solution added
- Repeat steps 2-5 for the same concentration twice more and calculate an average
- Repeat steps 2-6 for each of the known concentrations
- Results can be plotted as a line of best fit showing the volume of vitamin C needed to decolourise DCPIP against the concentration of vitamin C
- This is a calibration curve and can be used to find the concentration of vitamin C in unknown samples such as fruit juices
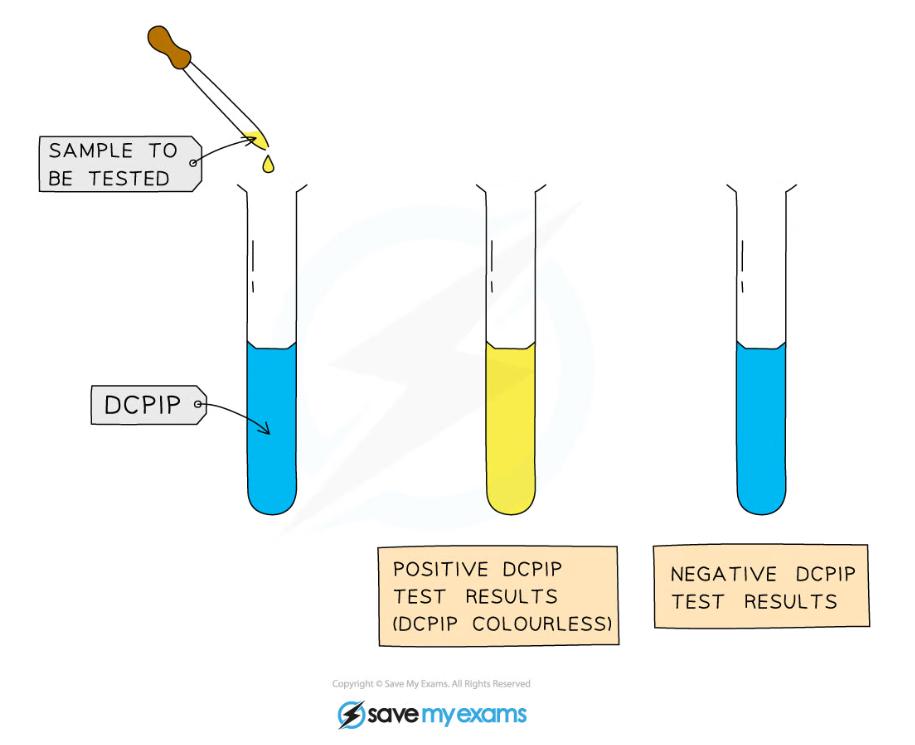
Drops of vitamin C solution of known concentration can be added to DCPIP to determine the volume required for the DCPIP to be decolourised
Risk assessment
- DCPIP is an irritant
- Avoid contact with the skin
- Wear eye protection
Results
- The volume of vitamin C solution required to decolourise DCPIP should decrease as the concentration of the vitamin C solution increases
- The results of the experiment can be plotted on a graph of volume of vitamin C needed to decolourise DCPIP against the concentration of vitamin C
- The line of best fit for such a graph is known as a calibration curve; unknown substances can be compared to it to gain an estimate of their vitamin C concentration
- This calibration curve produced from this experiment can be used to estimate the concentration of vitamin C in fruit juices
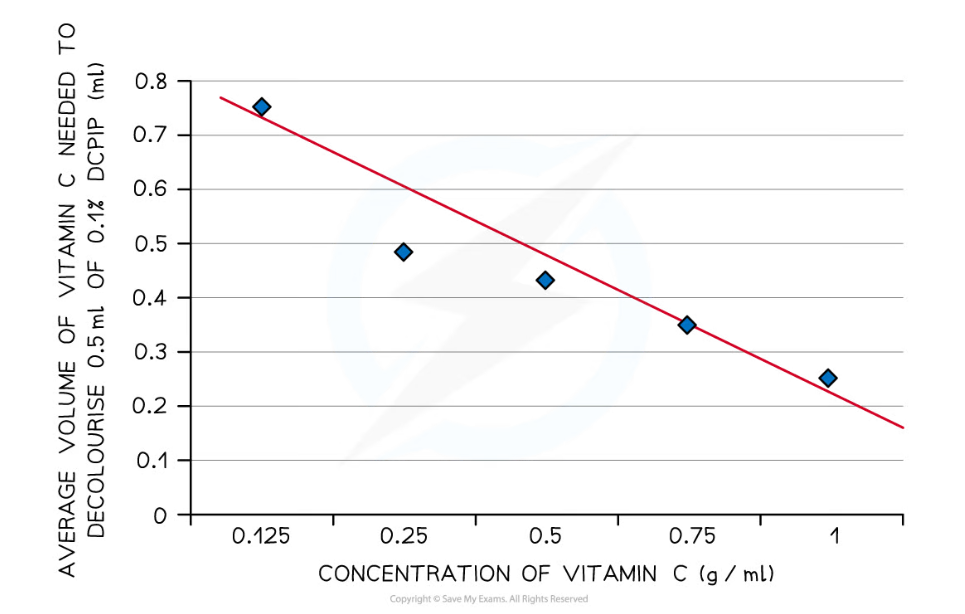
A graph of volume of vitamin C needed to decolourise DCPIP against vitamin C concentration can be used as a calibration curve to estimate the vitamin C concentration of unknown substances
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





