- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Physics: Double Science 复习笔记:7.1.6 Detecting Radiation
Edexcel IGCSE Physics: Double Science 复习笔记:7.1.6 Detecting Radiation
Detecting Radiation
- It is important to regulate the exposure of humans to radiation
- The amount of radiation received by a person is called the dose and is measured in sieverts (Sv)
- One sievert is a very big dose of radiation
- It would cause acute radiation poisoning
- People would normally receive about 3 mSv (0.003 Sv) in one year
- To protect against over-exposure, the dose received by different activities is measured
- Radiation can be measured and detected using a photographic film or a Geiger–Müller tube
Photographic Film
- Photographic films detect radiation by becoming darker when it absorbs radiation, just like it does when it absorbs visible light
- The more radiation the film absorbs, the darker it is when it is developed
- People who work with radiation, such as radiographers, wear film badges which are checked regularly to monitor the levels of radiation absorbed
- To get an accurate measure of the dose received, the badge contains different materials that the radiation must penetrate to reach the film
- These materials may include aluminium, copper, paper, lead and plastic
- The diagram shows what a typical radiation badge looks like:
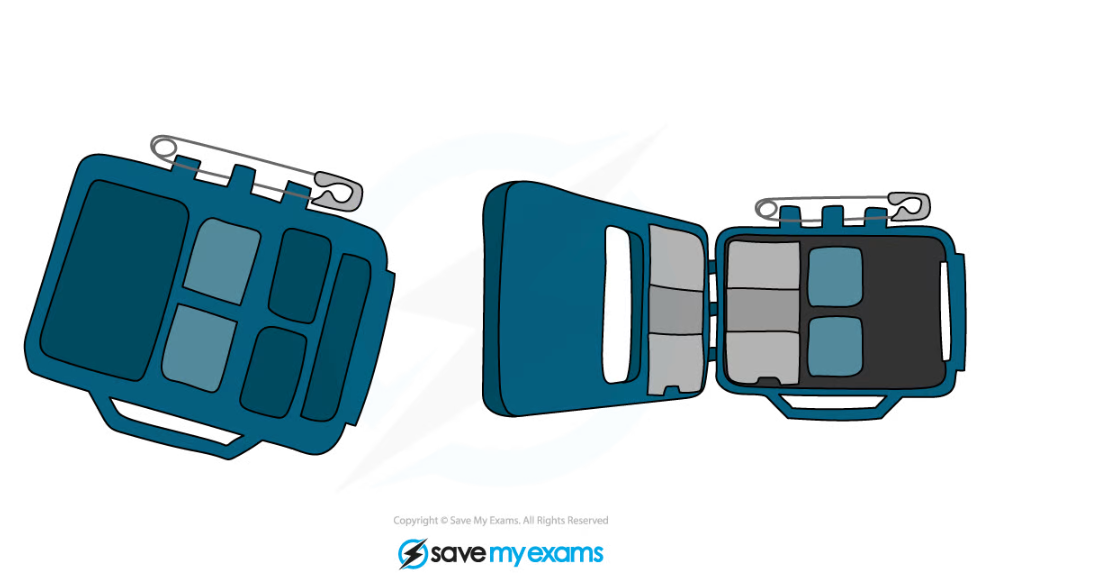
A badge containing photographic film can be used to monitor a person’s exposure to radiation
- The badge shows the amount of different types of radiation that the radiographer has been exposed to
- Different areas of the film are exposed to different types of radiation
- Alpha radiation is unlikely to be detected at all as it will be absorbed / stopped by the paper
- Beta radiation is absorbed by the aluminium
- Gamma (or X-rays) affect all areas of the film but the lead will reduce some of the gamma radiation
Geiger-Müller tube
- The Geiger-Müller tube is the most common device used to measure and detect radiation
- Each time it absorbs radiation, it transmits an electrical pulse to a counting machine
- This makes a clicking sound or displays the count rate
- The greater the frequency of clicks, or the higher the count rate, the more radiation the Geiger-Müller tube is absorbing
- Therefore, it matters how close the tube is to the radiation source
- The further away from the source, the lower the count rate detected
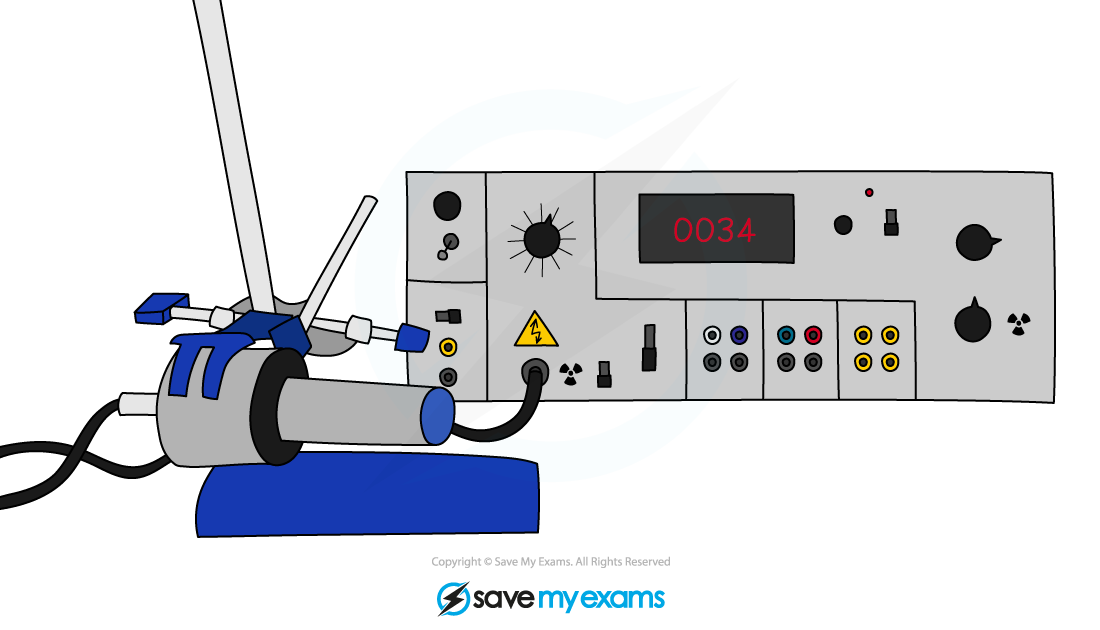
A Geiger-Müller tube (or Geiger counter) is a common type of radiation detector
Worked Example
A Geiger-Müller tube is used to detect radiation in a particular location. If it counts 16,000 decays in 1 hour, what is the count rate?
Step 1: Identify the different variables
-
- The number of decays is 16 000
- The time is 1 hour
Step 2: Determine the time period in seconds
-
- 1 hour is equal to 60 minutes, and 1 minute is equal to 60 seconds
Time period = 1 × 60 × 60 = 3600 seconds
Step 3: Divide the total counts by the time period in seconds
Counts ÷ Time period = 16 000 ÷ 3600 = 4.5
-
- Therefore, there are 4.5 decays per second
Exam Tip
If asked to name a device for detecting radiation, the Geiger-Müller tube is a good example to give. You can also refer to it as a GM tube, a GM detector, GM counter, Geiger counter etc. (The examiners will allow some level of misspelling, providing it is readable). Don’t, however, refer to it as a ‘radiation detector’ as this is too vague and may simply restate what was asked for in the question.
Background Radiation
- It is important to remember that radiation is a natural phenomenon
- Radioactive elements have always existed on Earth and in outer space
- However, human activity has added to the amount of radiation that humans are exposed to on Earth
- Background radiation is defined as:
The radiation that exists around us all the time
- There are two types of background radiation:
- Natural sources
- Man-made sources
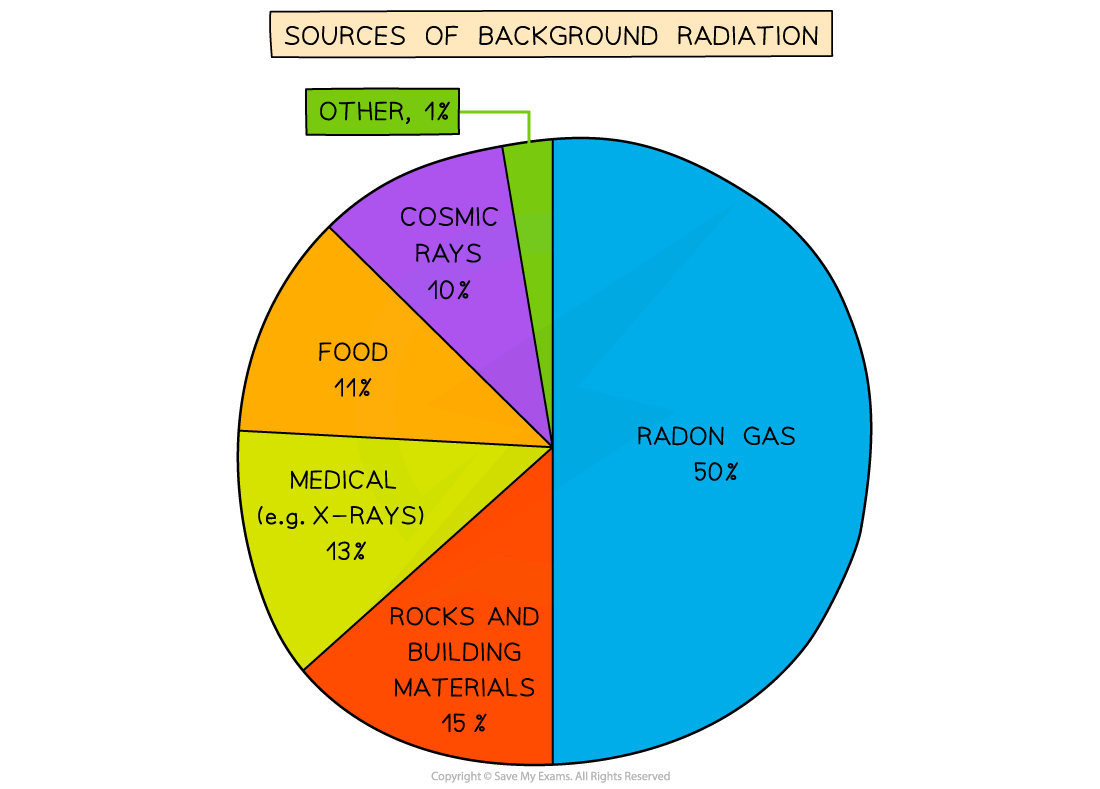
Background radiation is the radiation that is present all around in the environment. Radon gas is given off from some types of rock
- Every second of the day there is some radiation emanating from natural sources such as:
- Rocks
- Cosmic rays from space
- Foods
- Man-made sources of radiation increase the background radiation levels, examples include:
- Fallout from nuclear weapons testing and nuclear accidents
- Exposure from medical testing
Natural Sources
- Radon gas from rocks and soil
- Heavy radioactive elements, such as uranium and thorium, occur naturally in rocks in the ground
- Uranium decays into radon gas, which is an alpha emitter
- This is particularly dangerous if inhaled into the lungs in large quantities
- Cosmic rays from space
- The sun emits an enormous number of protons every second
- Some of these enter the Earth’s atmosphere at high speeds
- When they collide with molecules in the air, this leads to the production of gamma radiation
- Other sources of cosmic rays are supernovae and other high energy cosmic events
- Carbon-14 in biological material
- All organic matter contains a tiny amount of carbon-14
- Living plants and animals constantly replace the supply of carbon in their systems hence the amount of carbon-14 in the system stays almost constant
- Radioactive material in food and drink
- Naturally occurring radioactive elements can get into food and water since they are in contact with rocks and soil containing these elements
- Some foods contain higher amounts such as potassium-40 in bananas
- However, the amount of radioactive material is minuscule and is not a cause for concern
Man-Made Sources
- Medical sources
- In medicine, radiation is utilised all the time
- Uses include X-rays, CT scans, radioactive tracers, and radiation therapy
- Nuclear waste
- While nuclear waste itself does not contribute much to background radiation, it can be dangerous for the people handling it
- Nuclear fallout from nuclear weapons
- Fallout is the residue radioactive material that is thrown into the air after a nuclear explosion, such as the bomb that exploded at Hiroshima
- While the amount of fallout in the environment is presently very low, it would increase significantly in areas where nuclear weapons are tested
- Nuclear accidents
- Accidents such as that in Chernobyl contributed a large dose of radiation into the environment
- While these accidents are now extremely rare, they can be catastrophic and render areas devastated for centuries
Corrected Count Rate
- Background radiation must be accounted for when taking readings in a laboratory
- This can be done by taking readings with no radioactive source present and then subtracting this from readings with the source present
- This is known as the corrected count rate
Worked Example
A student is using a Geiger-counter to measure the counts per minute at different distances from a source of radiation. Their results and a graph of the results are shown here.
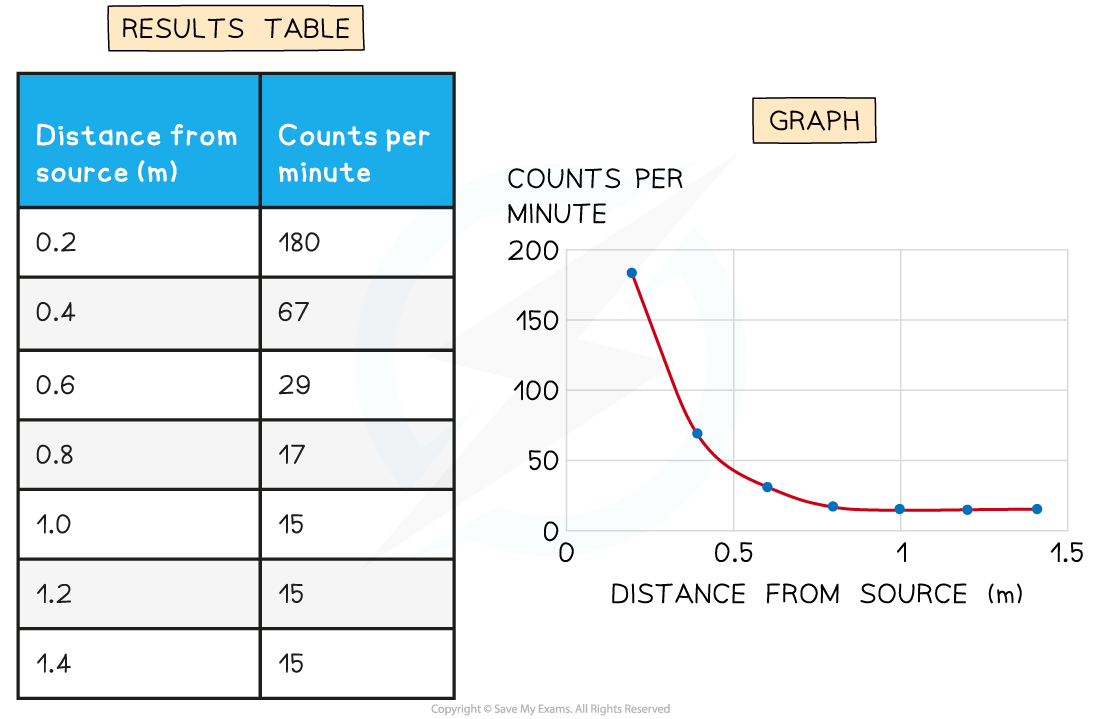 Determine the background radiation count.
Determine the background radiation count.
Step 1: Determine the point at which the source radiation stops being detected
-
- The background radiation is the amount of radiation received all the time
- When the source is moved back far enough it is all absorbed by the air before reaching the Geiger-counter
- Results after 1 metre do not change
- Therefore, the amount after 1 metre is only due to background radiation
Step 2: State the background radiation count
-
- The background radiation count is 15 counts per minute
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





