- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Physics: Double Science 复习笔记:7.1.2 Isotopes
Edexcel IGCSE Physics: Double Science 复习笔记:7.1.2 Isotopes
Isotopes
- Although the number of protons in a particular element is always the same, the number of neutrons can be different
- Isotopes are atoms of the same element that have an equal number of protons but a different number of neutrons
- In the diagram below are three isotopes of Hydrogen:
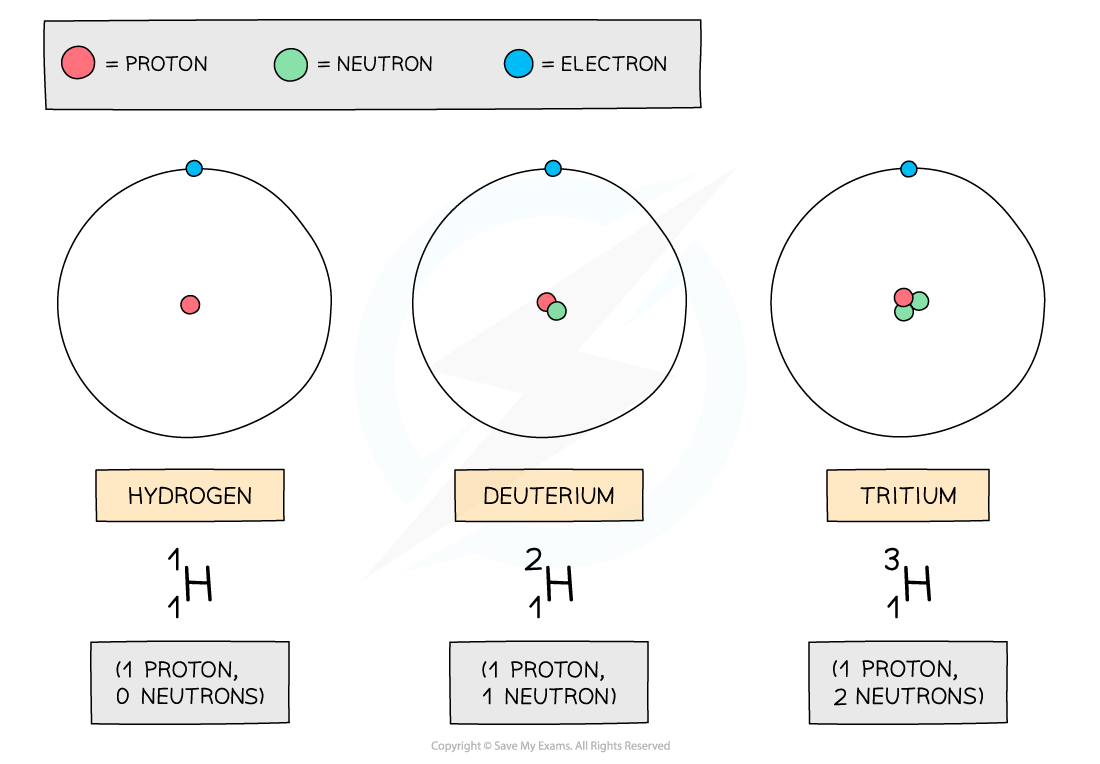
Hydrogen has three isotopes, each with a different number of neutrons
- Isotopes occur naturally, but some are more rare than others
- For example, about 2 in every 10,000 Hydrogen atoms is Deuterium
- Tritium is even more rare (about 1 in every billion billion hydrogen atoms)
Differences Between Isotopes
- The number of neutrons in an atom does not affect the chemical properties of an atom, such as its charge, but only its mass
- This is because neutrons have no charge but do have mass
- The charge of the nucleus of a particular element is always the same
- In the periodic table, the mass number of Chlorine is often given as 35.5
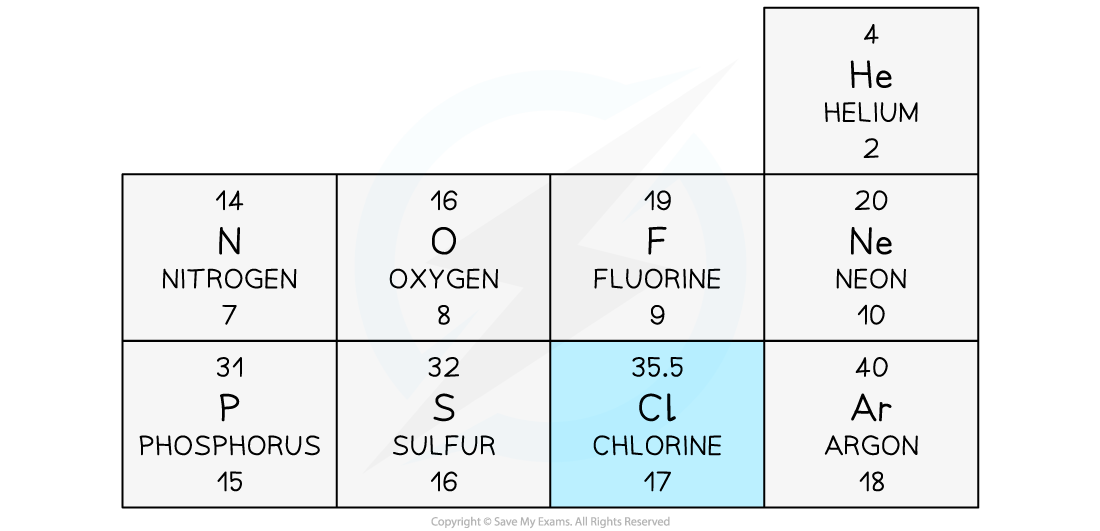
This section of a periodic table shows Chlorine as having a mass number of 35.5, but other elements have an integer mass number
- The mass number of Chlorine is given as 35.5 because it has roughly equal numbers of isotopes with a mass number of 35, and of 36
- The number of electrons and protons in different isotopes remains the same
- Isotopes tend to be more unstable due to the imbalance of protons and neutrons
Worked Example
State the number of protons, neutrons and electrons in Chlorine-35 and Chlorine-36 atoms.
Step 1: Determine the number of protons
-
- The atomic number is the number of protons
- Both Chlorine-35 and Chlorine-36 have 17 protons
Step 2: Determine the number of neutrons
-
- The mass number is the number of protons and neutrons
- Chlorine-35 neutrons: 35 - 17 = 18 neutrons
- Chlorine-36 neutrons: 36 - 17 = 19 neutrons
Step 3: Determine the number of electrons
-
- The number of electrons is equal to the number of protons
- Both Chlorine-35 and Chlorine-36 have 17 electrons
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





