- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Physics: Double Science 复习笔记:7.1.1 Atomic Structure
Edexcel IGCSE Physics: Double Science 复习笔记:7.1.1 Atomic Structure
Atomic Structure
- Atoms are the building blocks of all matter
- They are incredibly small, with a radius of only 1 × 10-10 m
- This means that about one hundred million atoms could fit side by side across your thumbnail
- Atoms have a tiny, dense nucleus at their centre, with electrons orbiting around the nucleus
- The radius of the nucleus is over 10,000 times smaller than the whole atom, but it contains almost all of the mass of the atom
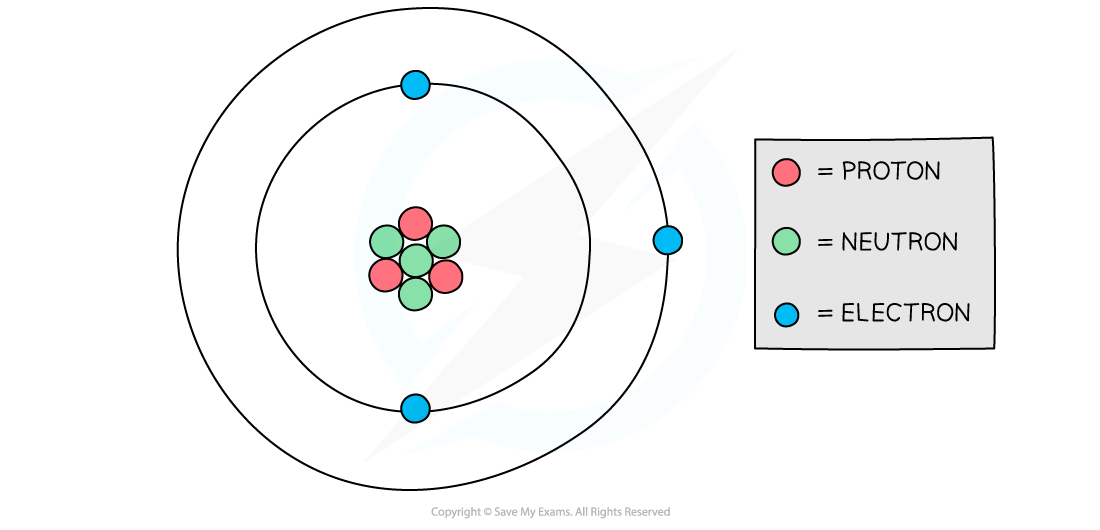
Diagram showing the structure of a Lithium atom. If drawn to scale then the electrons would be around 100 metres away from the nucleus!
Parts of the Atom
- The nucleus contains:
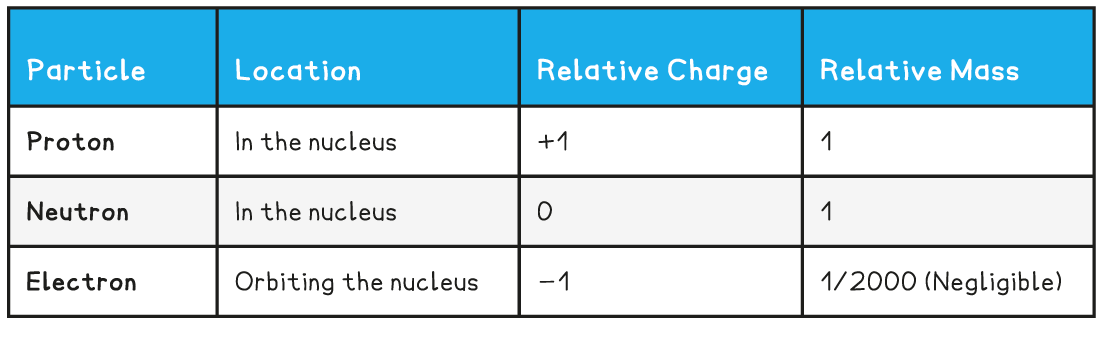
- Protons - positively charged particles with a relative atomic mass of one unit
- Neutrons – no charge, and also with a relative atomic mass of one unit
- Almost all of the atom is empty space, but moving around the nucleus there are:
- Electrons – negative charge with almost no mass (1/2000 the mass of a proton or neutron)
- The properties of each of the particles are shown in the table below:
Charge in the Atom
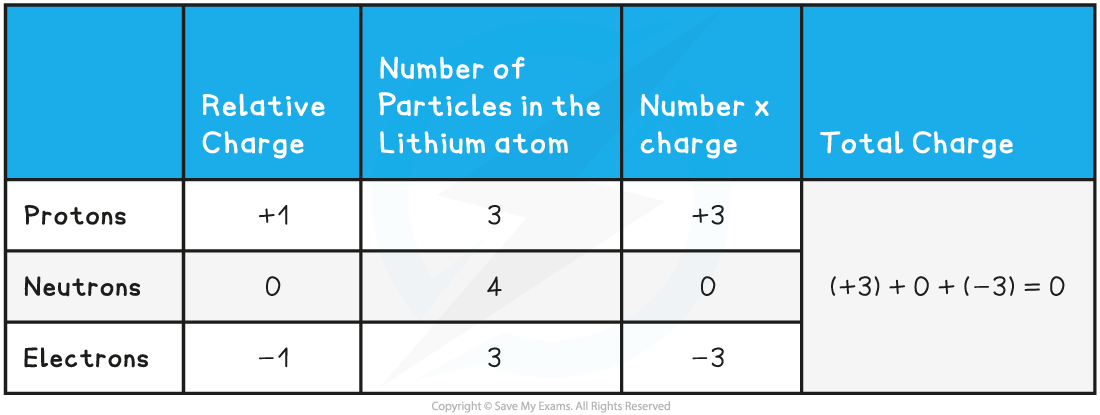
- Although atoms contain particles of different charge, the total charge within an atom is zero
- This is because the number of electrons is equal to the number of protons
- The following table sets out the calculation of the total charge in the Lithium atom in the diagram above:
Total Charge Calculation Table
- If an atom loses electrons, then it is said to be ionised
- Symbols are used to describe particular nuclear by their element symbol, atomic number and mass number
- This notation is called nuclear notation
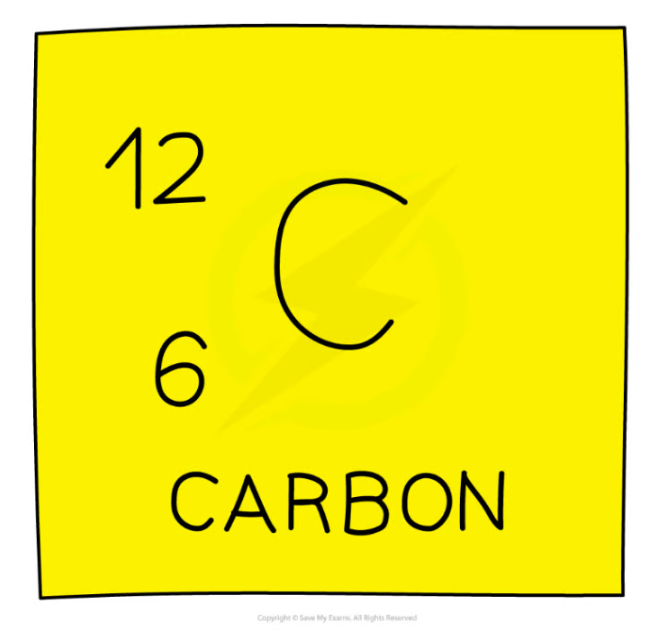
Carbon 12 in nuclear notation
Worked Example
A nucleus of carbon-12 is shown below.
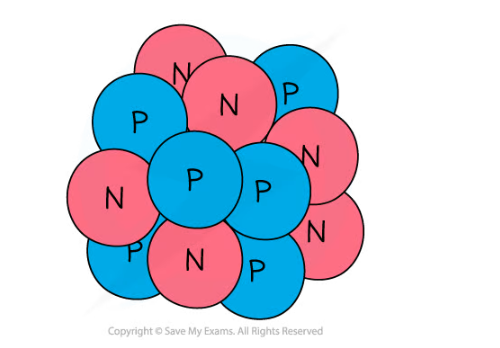
How many electrons are there in an atom of carbon-12?
Step 1: Count the number of protons in the carbon nucleus
-
- There are 6 protons in the carbon atom
Step 2: Determine the number of electrons
-
- Remember, the number of electrons in an atom is equal to the number of protons
- Therefore there must be 6 electrons in the carbon atom
Exam Tip
You may have noticed that the number of electrons is not part of the mass number. This is because electrons have a tiny mass compared to neutrons and protons. We say their mass is negligible when compared to the particles in the nucleus.
Atomic & Mass Number
Atomic Number
- The number of protons in an atom is called its atomic number (it can also be called the proton number)
- Elements in the periodic table are ordered by their atomic number
- Therefore, the number of protons determines which element an atom is
- The atomic number of a particular element is always the same
- For example:
- Hydrogen has an atomic number of 1. It always has just one proton
- Sodium has an atomic number of 11. It has 11 protons
- Uranium has an atomic number of 92. It has 92 protons
- The atomic number is also equal to the number of electrons in an atom
- This is because atoms have the same number of electrons and protons in order to have no overall charge
Mass Number
- The total number of particles in the nucleus of an atom is called its mass number (it can also be called the nucleon number)
- The mass number is the number of protons and neutrons in the atom
- The number of neutrons can be found by subtracting the atomic number from the mass number
Number of Neutron = Mass Number – Atomic Number
- For example, if a sodium atom has a mass number of 23 and an atomic number of 11, then the number of neutrons would be 23 – 11 = 12
Nuclear Notation
- The mass number and atomic number of an atom are shown by writing them with the atomic symbol
- This is called nuclear notation
- Here are three examples:
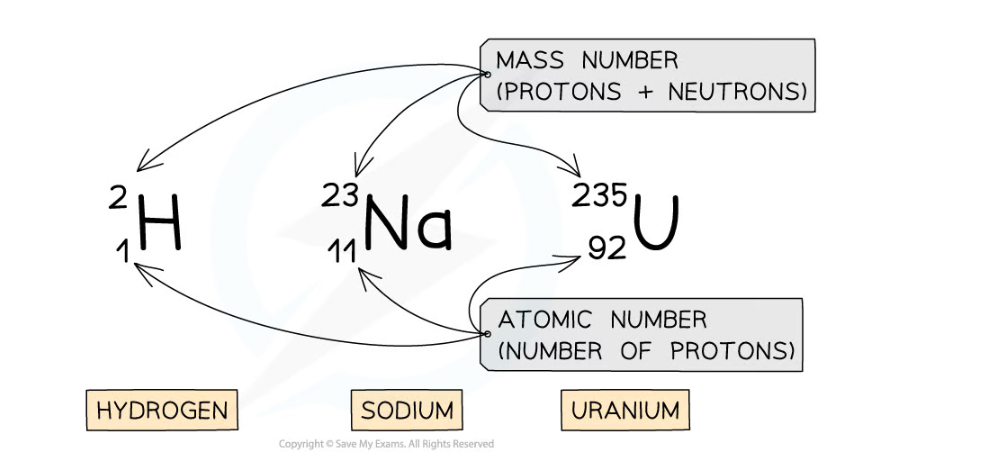
Examples of nuclear notation for atoms of Hydrogen, Sodium and Uranium
- The top number is the mass number
- This is equal to the total number of particles (protons and neutrons) in the nucleus
- The lower number is the atomic number
- This is equal to the total number of protons in the nucleus
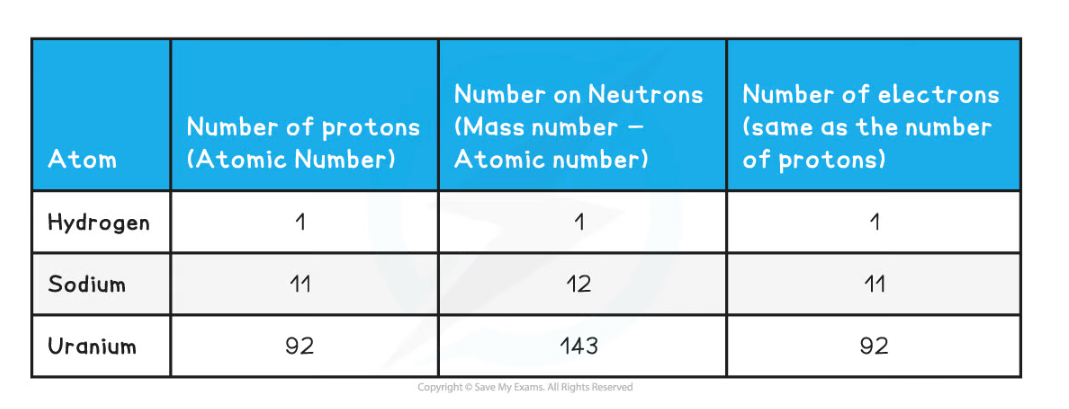
- The atomic and mass number of each type of atom in the examples above is shown in this table:
Number of Protons, Neutrons & Electrons Table
Worked Example
The element symbol for gold is Au. How many protons, neutrons and electrons are in the gold atom?
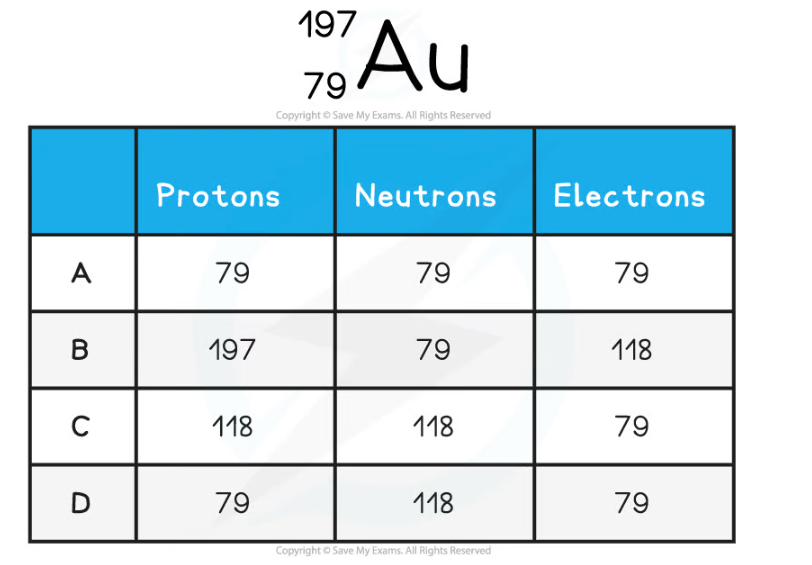
ANSWER: D
Step 1: Determine the atomic and mass number
-
- The gold atom has an atomic number of 79 (lower number) and a mass number of 197 (top number)
Step 2: Determine the number of protons
-
- The atomic number is equal to the number of protons
- The atom has 79 protons
Step 3: Calculate the number of neutrons
-
- The mass number is equal to the number of protons and neutrons
- The number of neutrons is equal to the mass number minus the atomic number
197 - 79 = 118
-
- The atom has 118 neutrons
Step 4: Determine the number of electrons
-
- An atom has the same number of protons and electrons
- The atom has 79 electrons
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





