- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记8.1.2 Electrochemical Cells
Core Practical 10: Investigating Electrochemical Cells
Measuring the EMF of a cell
- To measure a cell EMF you will need
- Two small beakers, around 75 cm3 capacity
- Strips of suitable metals such as copper, zinc, iron and silver
- 1.0 mol dm-3 solutions of the metal ions (nitrates, chlorides or sulfates depending on their solubility)
- A high resistance voltmeter (usually a digital multimeter has this)
- Two sets of wires with crocodile clips
- A salt bridge consisting of a strip of filter paper soaked in saturated potassium nitrate
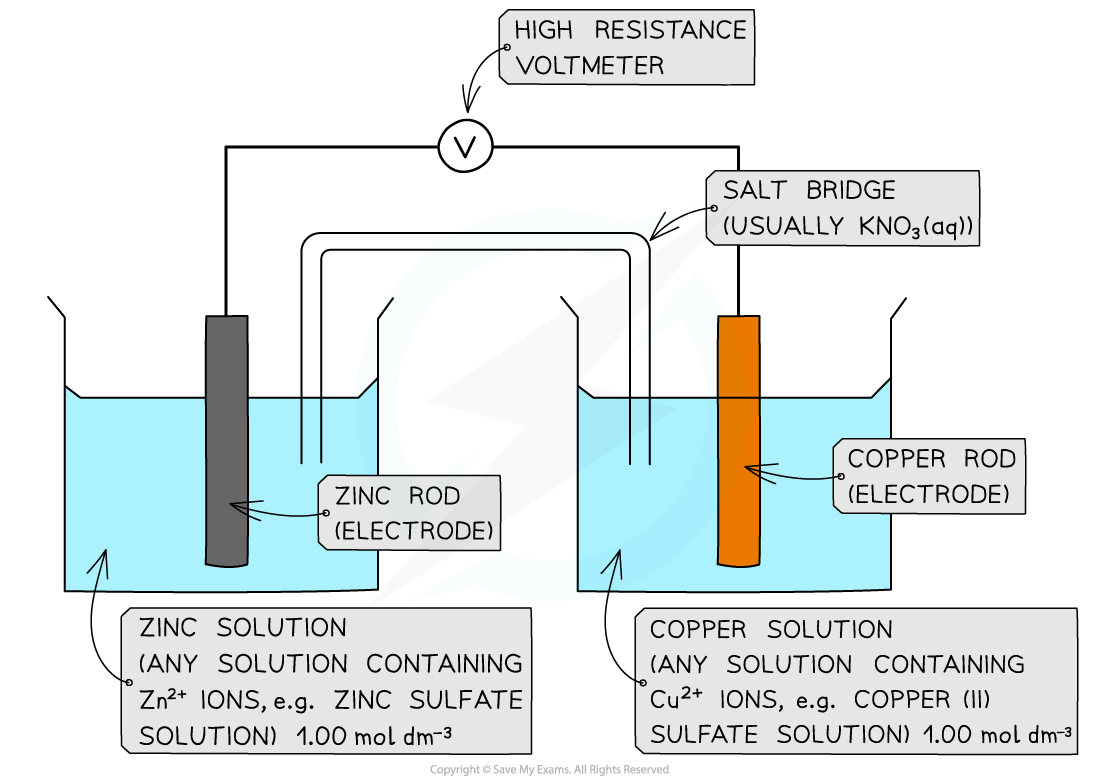
The experimental set up for measuring the EMF of a cell made of two metal / metal ion half cells
Steps in the procedure
- The strips of metals need to be freshly cleaned to remove any oxide coatings
- This can be done with a piece of sandpaper
- To support the metals, it is easiest to have long strips that can be folded over the side of the beaker and held in place with the crocodile clips
- Fill up the beakers to about two thirds of the way with the metal ion solutions
- Using tongs, dip a strip of filter paper into a beaker of saturated potassium nitrate solution and then place it between the two beakers making sure the ends of the strip are well immersed in the solutions
- Connect the crocodile clips to the voltmeter, wait for a steady reading and record the measurement
Practical tips
- If you don't get a positive reading on the voltmeter swap the terminals around
- Voltmeters will have marked positive and negative terminals (usually in red and black, respectively), so when you get a positive reading this tells you the relative polarity of the metals in the cell
- Change the salt bridge each time, to prevent cross contamination of ions between half cells
Specimen Results
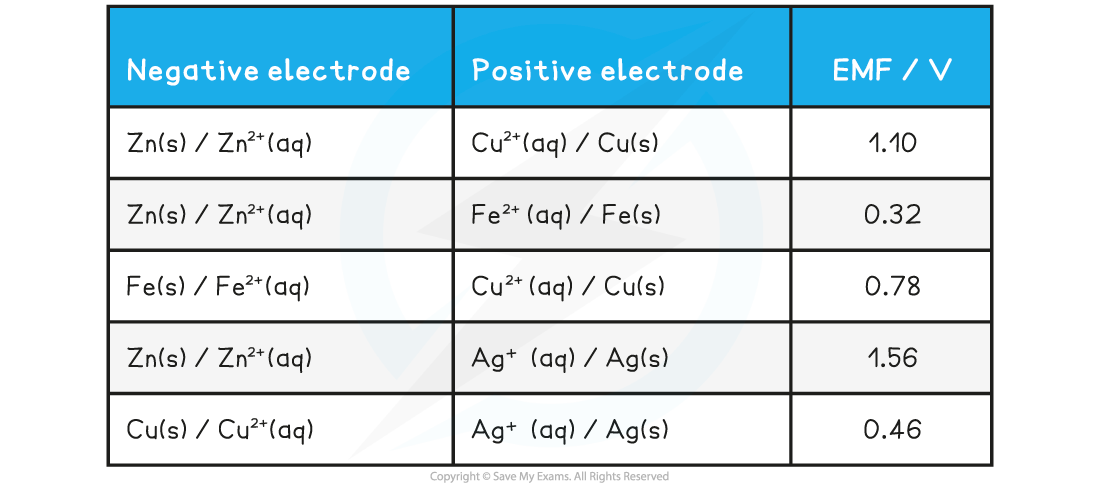
- Here is a set of typical results for this experiment
Specimen EMF Results (theoretical values) Table
Analysis
- It is unlikely you will get very close to the theoretical results as these would be obtained under standard conditions which are hard to achieve in a school laboratory
- However, the relative EMF of cells you construct should match the theoretical values
- The higher the EMF, the larger the difference in reactivity ('electron pushing power') between the metals
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





