- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记7.7.5 Other Chromatography
Types of Column Chromatography
Column Chromatography
- In column chromatography, the column is often a long vertical glass tube or in the laboratory a burette is suitable
- An inert solid (usually powdered silica gel or alumina) is the stationary phase which is placed in the column
- A liquid solvent phase, mobile phase, is added into the column until it is saturated with solvent
- Care should be taken when setting up the column because cracks in the stationary phase can lead to issues during separation
- The sample mixture is dissolved in the solvent and introduced at the top of the column
- A pipette is usually used to carefully add the dissolved sample to the top of the column
- The aim here is to add the sample without disturbing the surface of the column so that the sample runs from one level through the column
- Once the sample has been added, more solvent (eluent) is added on top of the sample
- As the solvent runs through, fresh solvent is added to the top of the column so that it does not dry out
- The sample flows through the column via gravity
- This process can be sped up by pushing the sample and mobile phase through the column
- In school laboratories, this can be achieved by attaching a gas syringe to the top of the chromatography column
- In industrial / research laboratories, this is achieved by attaching an air line to the top of the chromatography column
- The component with the greatest attraction / affinity to the stationary phase takes the longest time to flow through the column
- If the components are coloured, then they can be identified using the Rf value
- If the components are colourless, then other techniques such as fluorescence under UV light can be used to show their position in the column
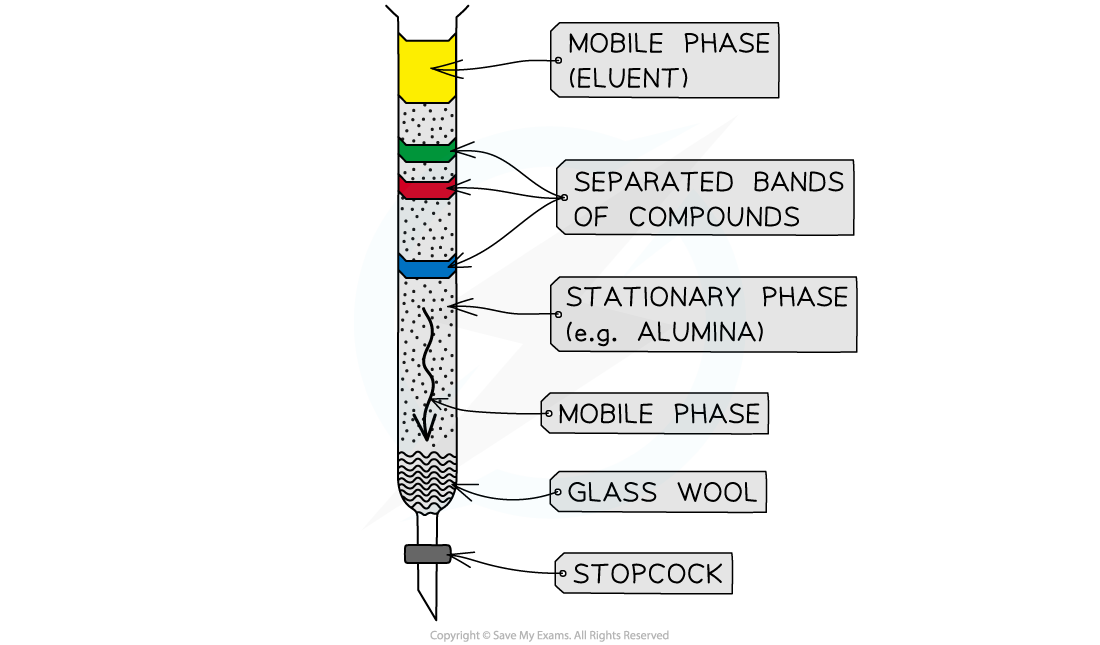
Column chromatography showing the separation of coloured compounds in a glass burette
High performance liquid chromatography, HPLC
- This is essentially the same as column chromatography
- The main differences are that:
- The column doesn't work via gravity, the sample is pumped through by the solvent
- The particles of the stationary phase are much smaller, leading to greater separation of compounds
- There is a detector at the end of the column which measures retention time
- Retention time is the time taken from the sample being injected to the sample being detected
- HPLC is automated so the results are obtained quicker
- The HPLC equipment typically includes a computer which allows for quicker analysis and comparison of results against known compounds in a database
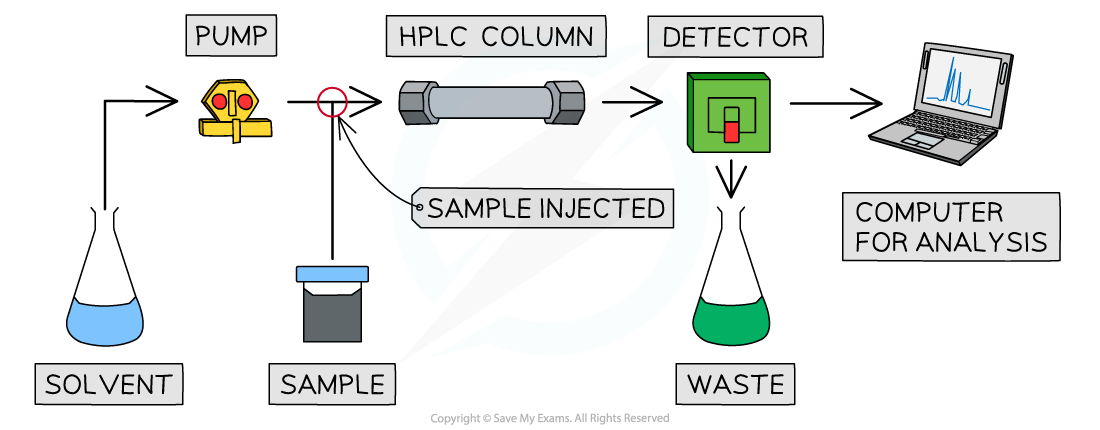
Gas-Liquid Chromatography, GLC
- Gas-Liquid Chromatography (GLC) is used for analysing:
- Gases
- Volatile liquids
- Solids in their vapour form
- The stationary phase:
- This method uses a long coiled column for the stationary phase
- Normally a non-volatile liquid is the stationary phase in GLC
- The Mobile phase
- An inert carrier gas (e.g. helium, nitrogen) moves the sample molecules through the stationary phase
- The sample is injected into the column through a self-sealing disc and the vapour formed is carried through the stationary phase using the inert-gas mobile phase
Retention times
- Once sample molecules reach the detector, their retention times are recorded
- This is the time taken for a component to travel through the column
- It depends upon the attraction between the solute and the stationary and mobile phases as well as the volatility and nature of the solute
- The retention times are recorded on a chromatogram where each peak represents a volatile compound in the analysed sample
- The relative sizes (i.e. areas) of the peaks are related to how much of each compound is present in the mixture
- Retention times are then compared with data book values to identify unknown molecules
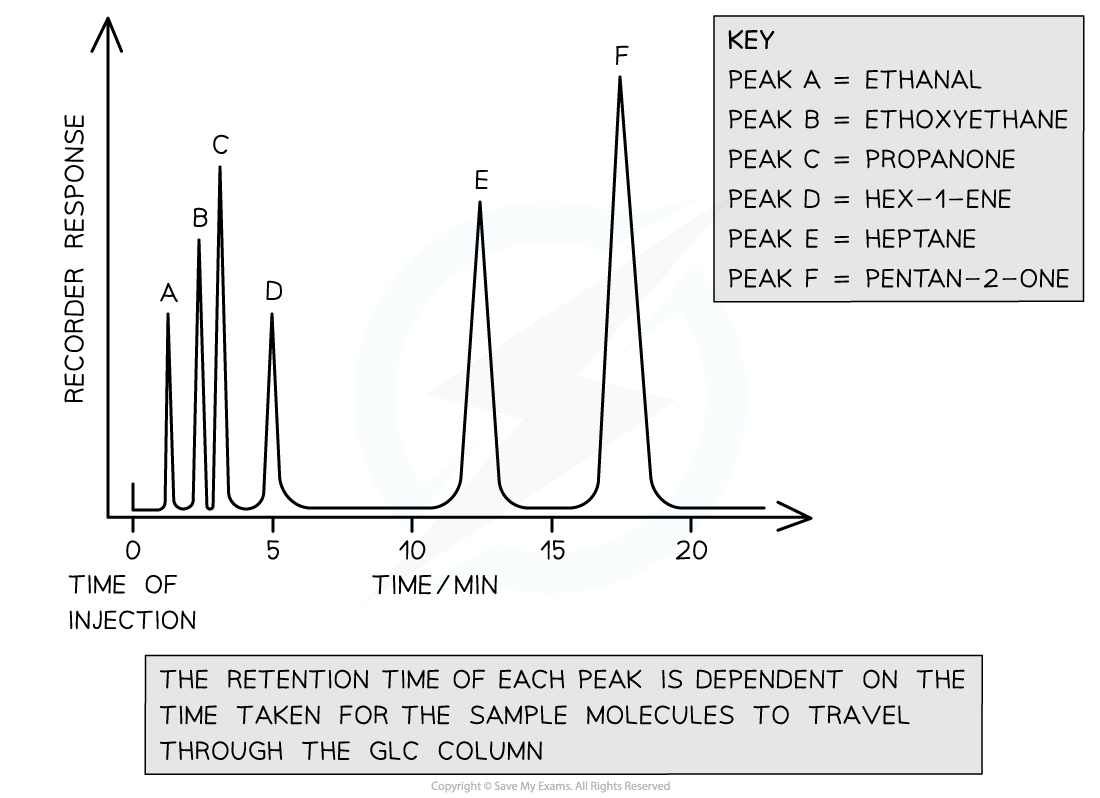
A gas chromatogram of a volatile sample compound has six peaks. Depending on each molecule’s interaction with the stationary phase, each peak has its own retention time
Worked Example
Analysis of a compound by GLC shows the presence of four components, A, B, C and D.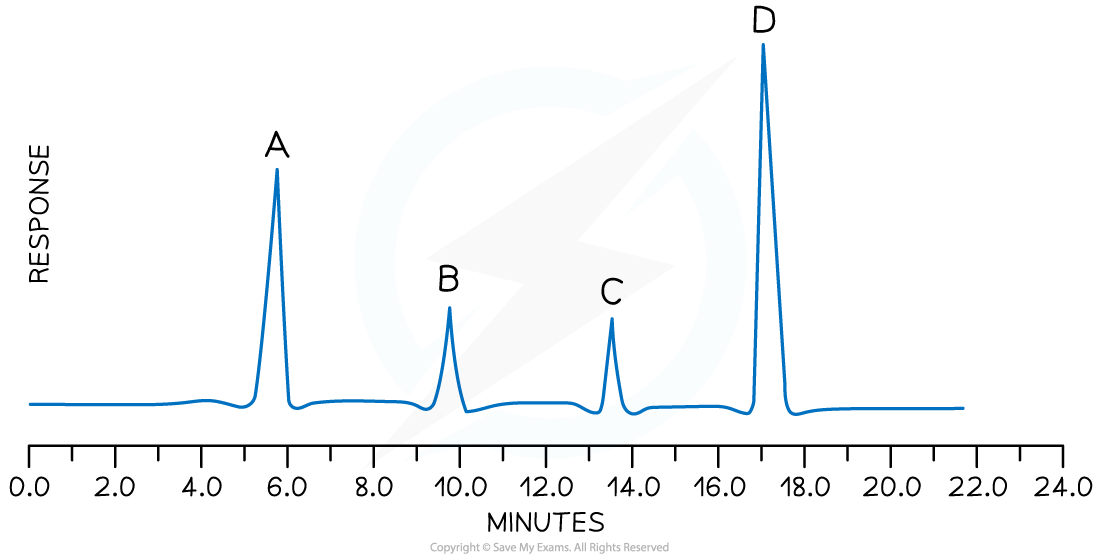
i) Which compound is present in the greatest quantity?
ii) Which compounds were present in equal amounts?
iii) Which compound had the strongest interaction with the stationary phase?
Answers:
i) D (the larger the relative size of the peak, the greater the quantity of that substance present)
ii) B and C (the peak sizes are equal)
iii) D (the larger the retention time, the greater the interaction of that component with the stationary phase)
Uses and limitations of HPLC and GLC
- HPLC and GLC are used to separate small amounts of components from a mixture
- They are often used to:
- Provide forensic evidence
- Drug testing, particularly in sports
- Analysis of environmental pollution
- Detecting explosives in baggage
- However, they are not very useful at identifying specific compounds, in legal terms - positive identification or beyond reasonable doubt
- This is because:
- Different compounds may have the same retention time
- It can be difficult to manage the conditions, e.g. temperature and pressure
- Some unknown compounds may not have a reference for comparison in the databases
- For this reason, HPLC and GLC are often coupled with other analytical techniques, most commonly mass spectrometry
- This results in HPLC-MS and GC-MS (GLC is sometimes abbreviated to GC)
- This means that components can be separated from mixtures and then analysed all within one machine
Problems with drug testing
- GC-MS is the most common method of drug detection in sports due to the accepted reliability of the results
- Even then, there can be problems
- One publicised problem is around the use of anabolic steroids
- Anabolic steroids can be used by athletes to improve muscle growth, increase production of red blood cells and strengthen bones by increasing their density
- They are also used to treat conditions such as osteoporosis, anaemia and some cancers
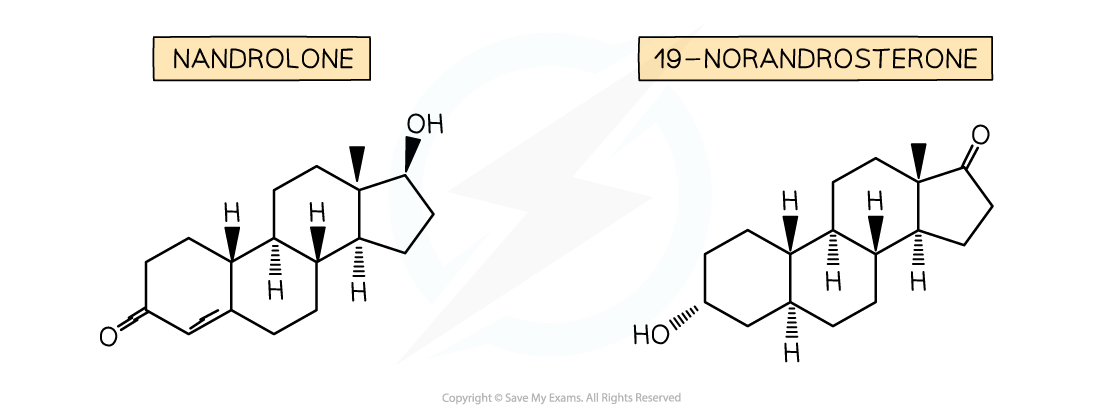
- One high profile anabolic steroid is nandrolone which is metabolised into a similar chemical called 19-norandrosterone
- Competitors in the Olympic Games are routinely urine tested for the presence of 19-norandrosterone
- A urine content above 2 nanograms per cm3 (0.000000002 g per cm3) is a positive test and can result in the athlete being disqualified and risking further sanctions
- There is debate about nandrolone due to its genuine medical applications and the fact that it may be in some nutritional and dietary supplements
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





