- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记7.5.5 Amide Chemistry
Amide Formation
- Amides are organic compounds with an -CONR2 functional group
- They can be prepared from the condensation reaction between an acyl chloride and ammonia or amine
- In a condensation reaction, two organic molecules join together and in the process eliminate a small molecule
- In this case, the acyl chlorides and ammonia or amine join together to form an amide and eliminate an HCl molecule
Condensation reaction
- The chlorine atom in acyl chlorides is electronegative and draws electron density from the carbonyl carbon
- The carbonyl carbon is therefore electron-deficient and can be attacked by nucleophiles
- The nitrogen atom in ammonia and amines has a lone pair of electrons which can act as a nucleophile and attack the carbonyl carbon
- As a result, the C-Cl bond is broken and an amide is formed
- Whether the product is a substituted amide or not, depends on the nature of the nucleophile
- Primary and secondary amines will give a substituted amide
- The reaction of acyl chlorides with ammonia will produce a non-substituted amide
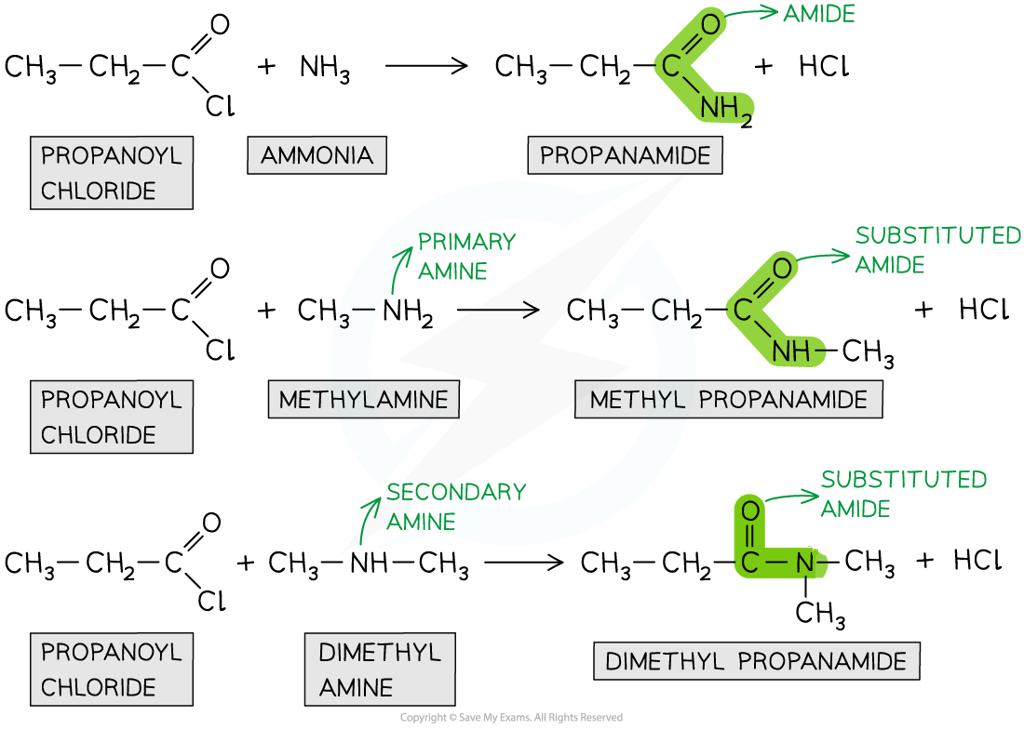
Acyl chlorides undergo condensation reactions with ammonia and amines to form amides
- Note that ammonia is basic and the inorganic product is acidic, so there will be a reaction between the two molecules
NH3 + HCl → NH4Cl
- We can therefore write the overall equation for the reaction of propanoyl chloride and ammonia as
CH3CH2COCl + 2NH3 → CH3CH2CONH2 + NH4Cl
Polyamide Formation
Amide link
- Polyamides are also formed using condensation polymerisation
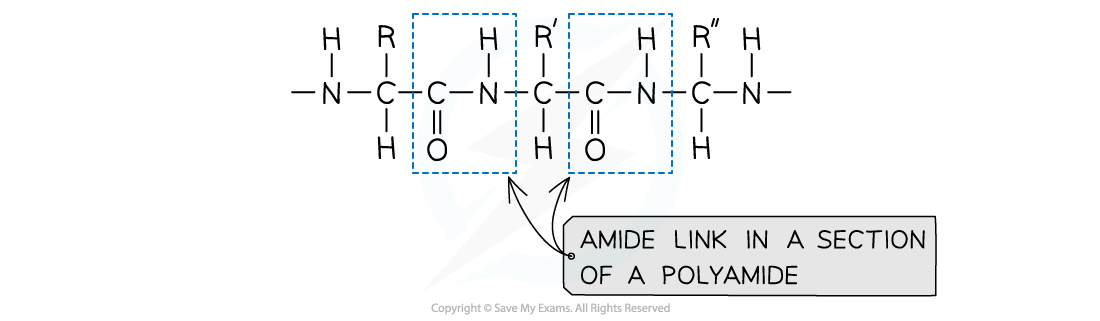
An amide link - also known as a peptide link - is the key functional group in a polyamide
Monomers
- A diamine and a dicarboxylic acid are required to form a polyamide
- A diamine contains 2 -NH2 groups
- A dicarboxylic acid contains 2 -COOH groups
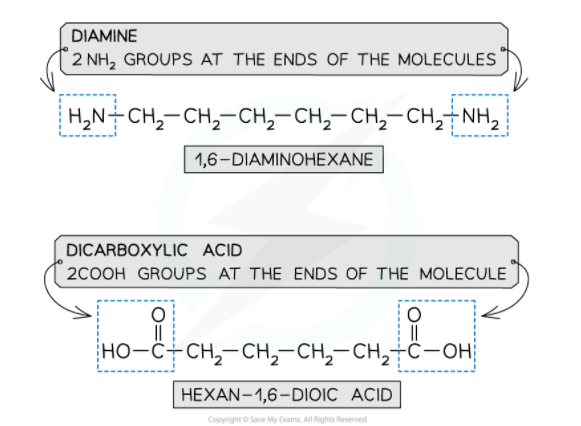
The monomers for making polyamides
Formation of polyamides
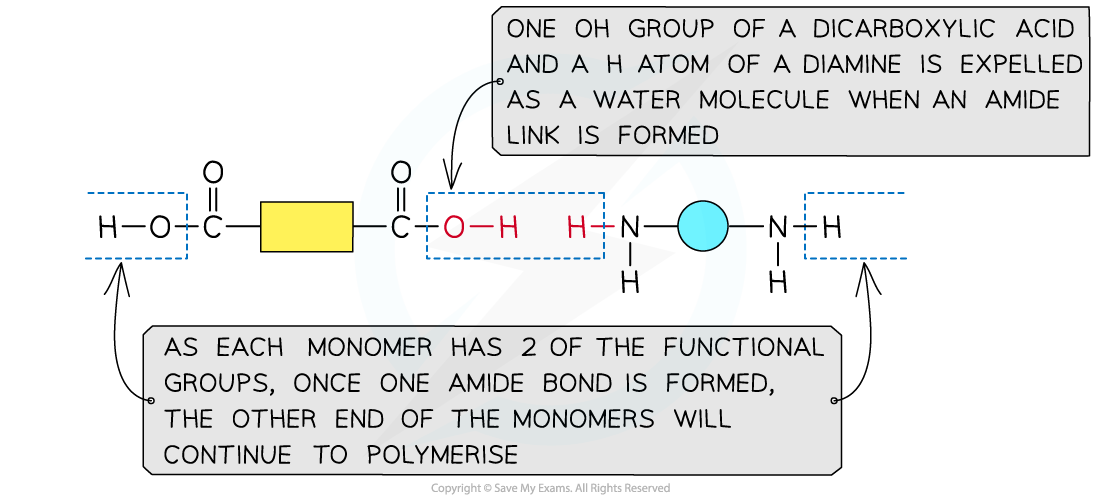
This shows the expulsion of a small molecule as the amide link forms
- Nylon 6,6 is a synthetic polyamide
- Its monomers are 1,6-diaminohexane and hexane-1,6-dioic acid
- The ‘6,6’ part of its name arises from the 6 carbon atoms in each of Nylon 6,6 monomers
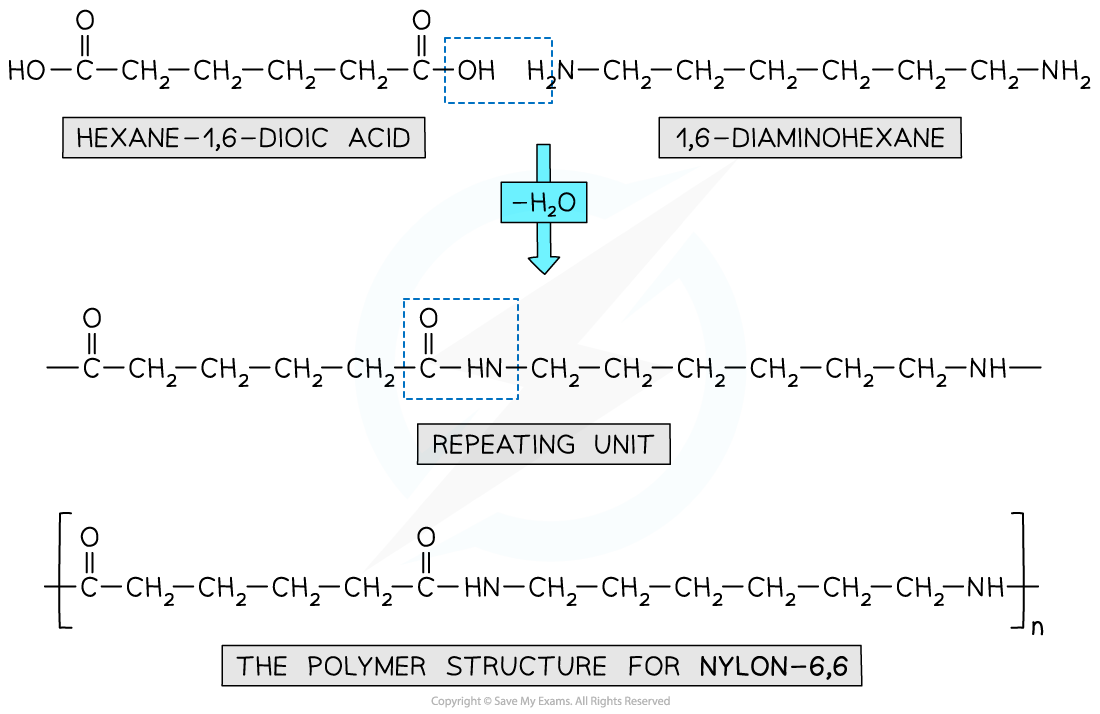
Nylon 6,6 is a synthetic polyamide made using diamine and dicarboxylic acid monomers
Kevlar
- Kevlar is another example of a polymer formed through condensation polymerisation
- The polymer chains are neatly arranged with many hydrogen bonds between them
- This results in a strong and flexible polymer material with fire resistance properties
- These properties also lend Kevlar to a vital application in bullet-proof vests
- The monomers used to make Kevlar
- 1,4-diaminobenzene
- Benzene-1,4-dicarboxylic acid
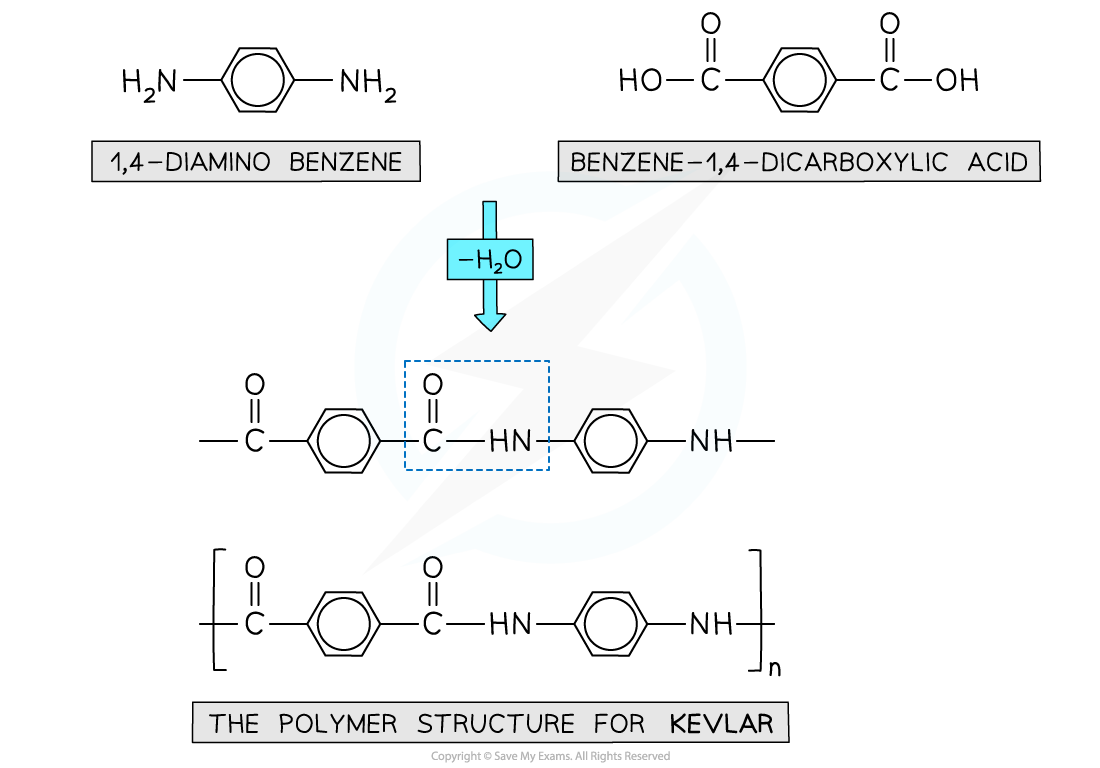
Kevlar is made using a diamine and dicarboxylic acid monomers
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





