- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记7.4.3 Electrophilic Substitution
Electrophilic Substitution
Reactions of Benzene
- The main reactions which benzene will undergo include the replacement of one of the 6 hydrogen atoms from the benzene ring
- This is different to the reactions of unsaturated alkenes, which involve the double bond breaking and the electrophile atoms 'adding on' to the carbon atoms
- These reactions where at least one of the H atoms from benzene are replaced, are called electrophilic substitution reactions
- The hydrogen atom is substituted by the electrophile
- You must be able to provide the mechanisms for specific examples of the electrophilic substitution of benzene
General Electrophilic Substitution Mechanism:


- The delocalised π system is extremely stable and is a region of high electron density
- Electrophilic substitution reactions involve an electrophile, which is either a positive ion or the positive end of a polar molecule
- There are numerous electrophiles which can react with benzene
- However, they usually cannot simply be added to the reaction mixture to then react with benzene
- The electrophile has to be produced in situ, by adding appropriate reagents to the reaction mixture
- The electrophilic substitution reaction in arenes consists of three steps:
- Generation of an electrophile
- Electrophilic attack
- Regenerating aromaticity
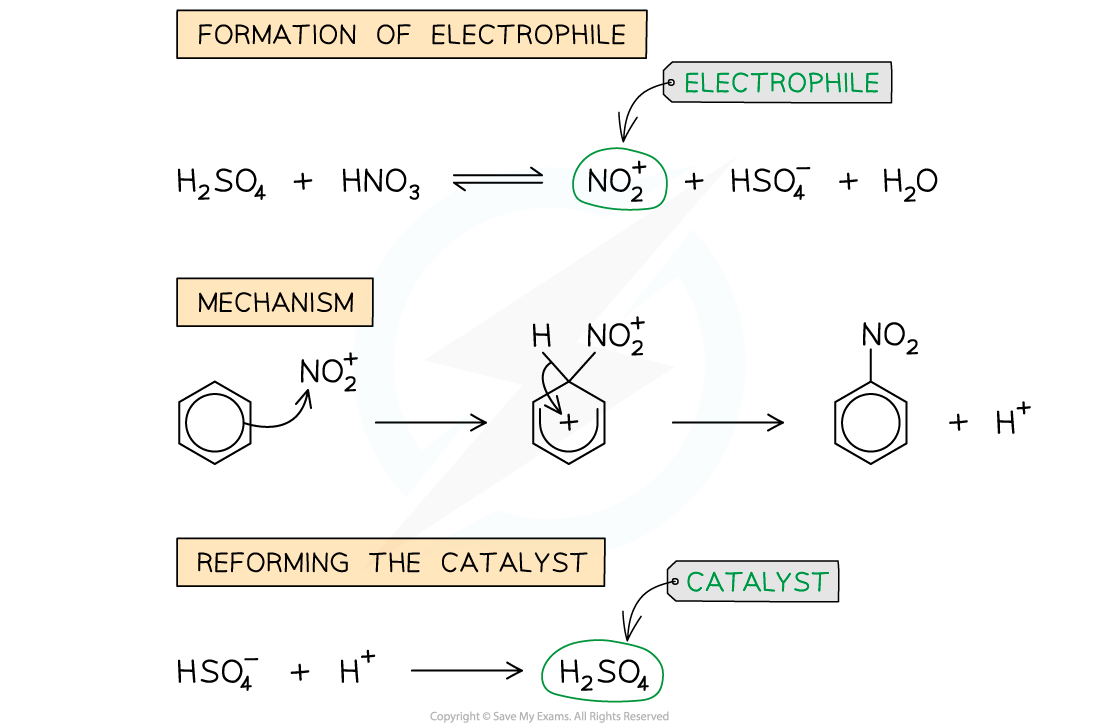
Nitration of benzene mechanism
- In the first step, the electrophile is generated
- The electrophile NO2+ ion is generated by reacting concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4)
Halogenation of benzene mechanism
- Benzene will undergo a substitution reaction with a halogen if a metal halide carrier is present
- This generates the electrophile for the reaction to occur
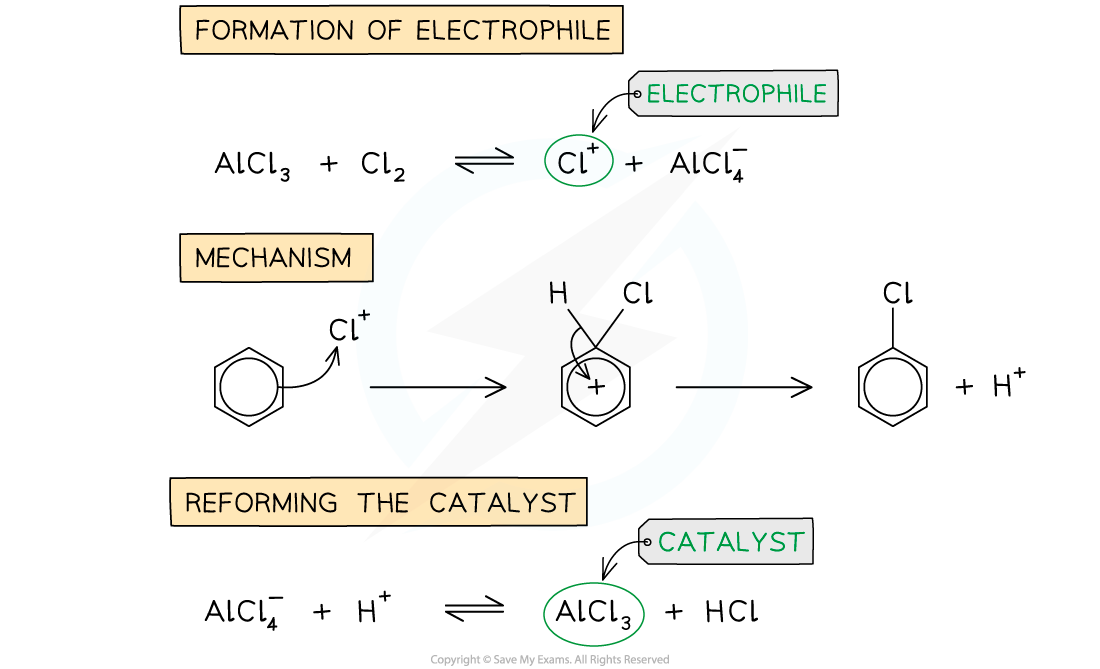
The different stages in the chlorination of benzene
Friedel-Crafts acylation mechanism
- In the Friedel-Crafts acylation reaction, an acyl group is substituted into the benzene ring
- An acyl group is an alkyl group containing a carbonyl, C=O group
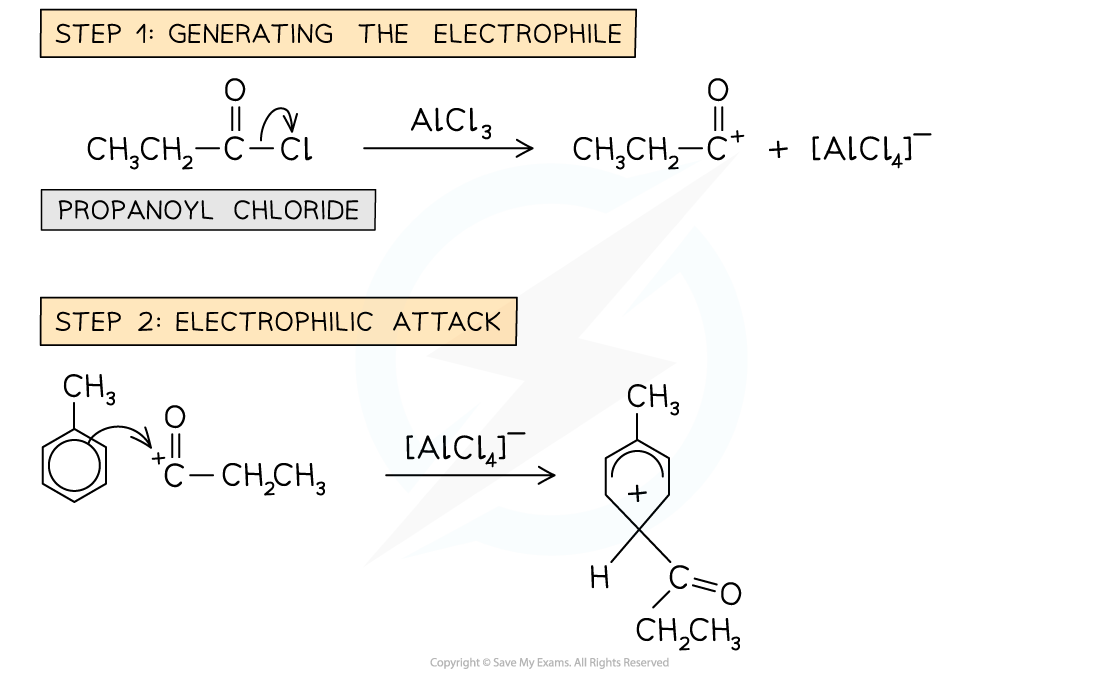
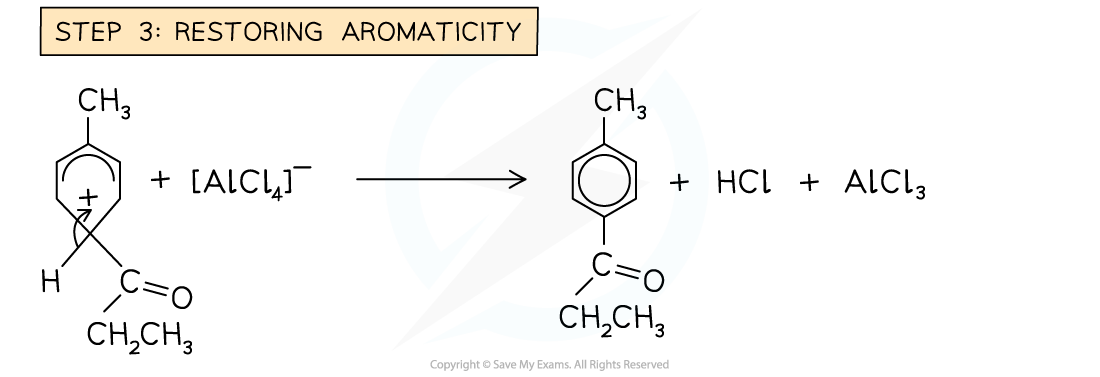
Example of a Friedel-Crafts acylation reaction
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





