- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记7.4.2 Reactions of Benzene
Reactions of Benzene
Reaction with oxygen
- Hydrocarbons will burn in air or oxygen to produce carbon dioxide and water providing sufficient oxygen is available
- Benzene reacts will also follow this pattern
2C6H6 (l) + 15O2 (g) → 12CO2 (g) + 6H2O (g)
- Given that a large volume of oxygen is required for this reaction, incomplete combustion could occur
- Therefore unreacted benzene may remain
- This would lead to a smokey yellow flame as there would be insufficient oxygen available
Halogenation
- The nature of benzene is different to other unsaturated compounds such as alkenes and halogenation via electrophilic addition is not possible
- Therefore aromatic compounds will react with halogens in the presence of a metal halide carrier
- iron(III) bromide
- aluminium chloride
- The reaction of the metal halide carrier acts as catalyst and creates the electrophile, X+(where X represents a halogen atom)
- At the end of the reaction it is regenerated
AlCl3 + Cl2 → AlCl4- + Cl+
FeBr3 + Br2 → FeBr4- + Br+
- The overall equation for halogenation is
C6H6 + X2 → C6H5X + HX
Or with Br2 in the presence of a AlBr3
C6H6 + Br2 → C6H5Br + HBr

Bromination of benzene
- Remember that one hydrogen atom on the benzene ring has been substituted for one halogen atom, therefore HX will be a product
Nitration
- Another example of a substitution reaction is the nitration of arenes
- In these reactions, a nitro (-NO2) group replaces a hydrogen atom on the arene
- The benzene is reacted with a mixture of concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4) at a temperature between 25 and 60 oC

Nitration of benzene
Friedel-Crafts Reactions
- Friedel-Crafts reactions are also substitution reactions
- Due to the aromatic stabilisation in arenes, they are often unreactive
- To use arenes as starting materials for the synthesis of other organic compounds, their structure, therefore, needs to be changed to turn them into more reactive compounds
- Friedel-Crafts reactions can be used to substitute a hydrogen atom in the benzene ring for an alkyl group (Friedel-Crafts alkylation) or an acyl group (Friedel-Crafts acylation)
- Like any other electrophilic substitution reaction, the Friedel-Crafts reactions consist of three steps:
- Generating the electrophile
- Electrophilic attack on the benzene ring
- Regenerating aromaticity of the benzene ring
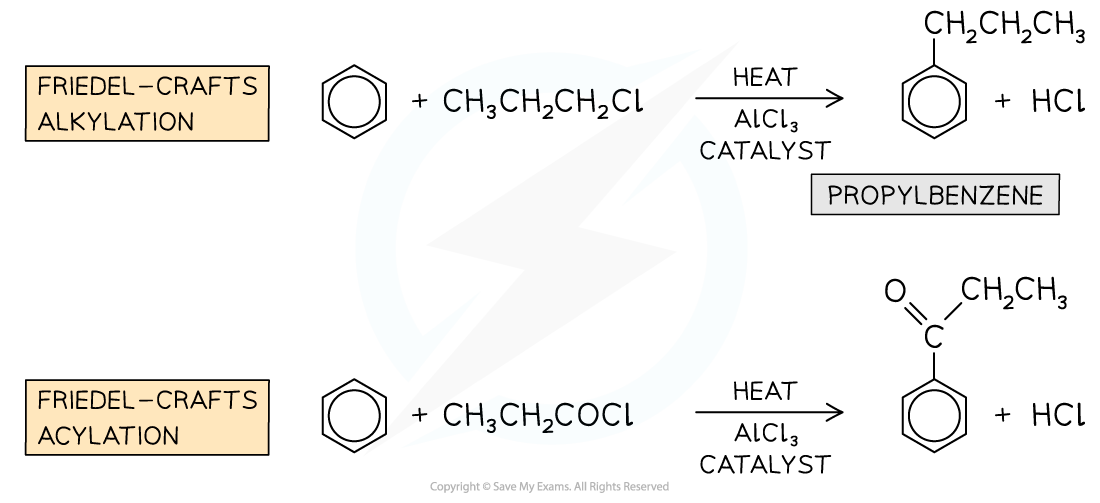
Examples of Friedel-Crafts alkylation and acylation reactions
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





