- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记6.2.8 Haemoglobin
Haemoglobin
- Haemoglobin is one of nature's complexes using a transition metal ion
- The haem molecule is a complex with iron(II) at its centre
- The haemoglobin complex contains a multidentate ligand made up of four haem groups
- These consist of mostly carbon and hydrogen atoms
- Each haem group has a nitrogen atom forming a dative covalent bond to the Fe2+ ion in a square planar complex
- There is a fifth dative bond from the protein (globin) to the Fe2+ ion
- Oxygen atoms form a dative covalent bond with the iron(II) which enables oxygen molecules to be transported around the body in the blood
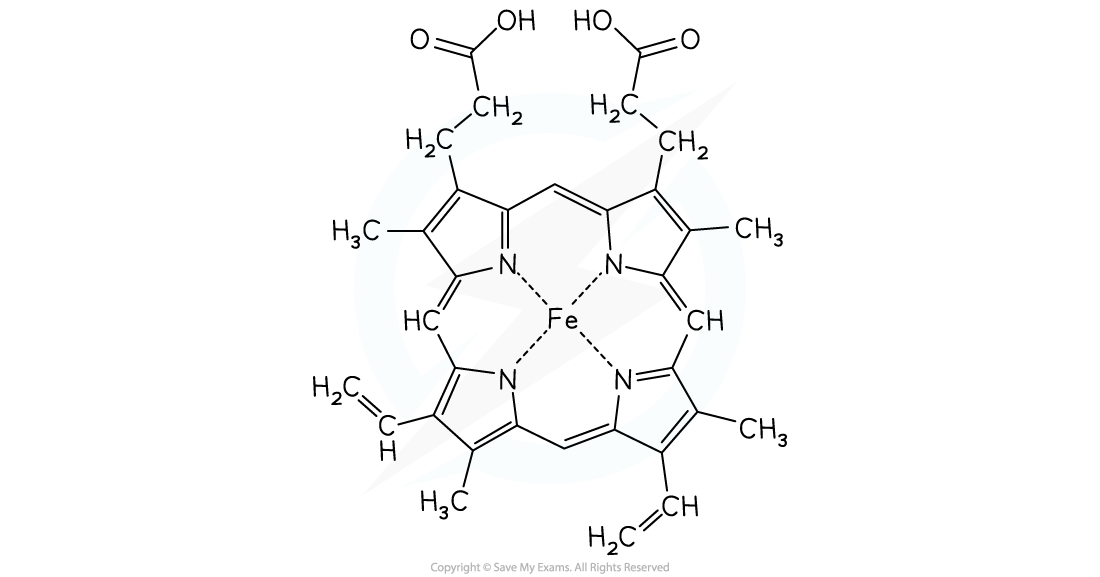
The haem molecule with iron(II) at its centre
- Oxygen molecules are not very good ligands and bond weakly to the iron(II)
- The weak bonds allows them to break off easily and be transported into cells
Exam Tip
You do not need to be familiar with the structure of the haem group
Ligand Exchange in Haemoglobin
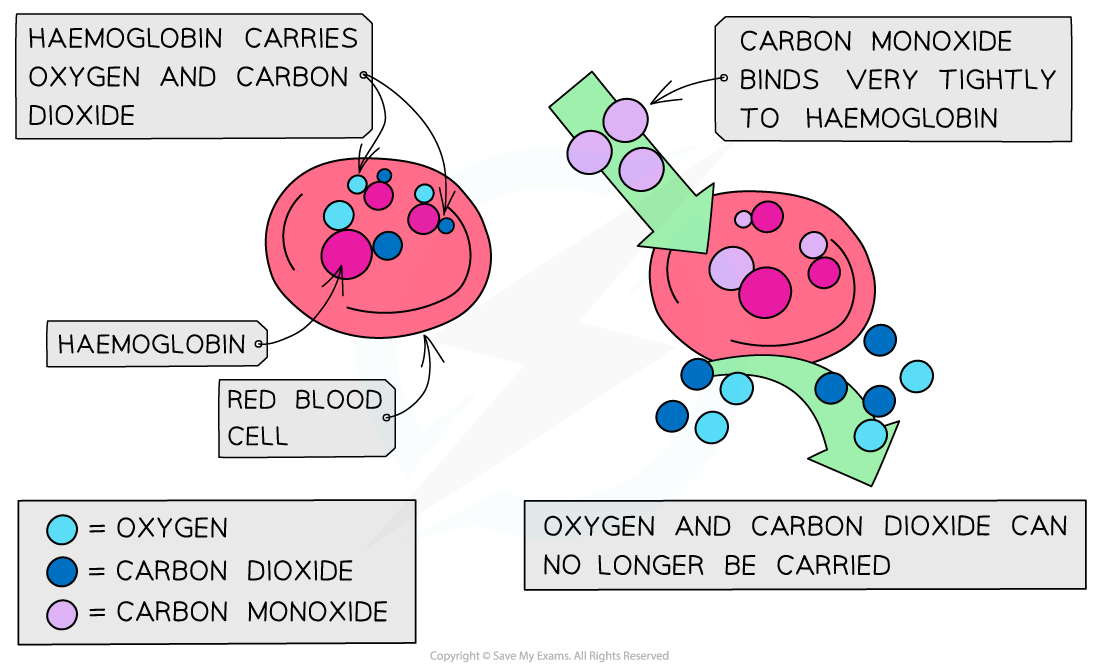
- Carbon monoxide is toxic because it is a better ligand than oxygen and binds strongly and irreversibly to the iron(II) preventing oxygen from being carried to the cells
- If oxygen attached to the haemoglobin (oxyhaemoglobin) is replaced by carbon monoxide (carboxyhaemoglobin), a darker red colour is produced in the haem complex
- A sign of carbon monoxide poisoning
- The condition anaemia occurs when a person does not have enough haemoglobin in their blood due to a loss of blood or deficiency in iron
- Deficiency in iron can be restored by taking iron sulfate tables in the diet
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





