- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记6.2.2 Transition Metal Complexes
Transition Metal Complexes
- Transition element ions can form complexes which consist of a central metal ion and ligands
- A ligand is a molecule or ion that forms a co-ordinate bond with a transition metal by donating a pair of electrons to the bond
- This is the definition of a Lewis base - electron pair donor
- This means ligands have a negative charge or a lone pair of electrons capable of being donated
- This definition may seem familiar: a ligand is the same as a nucleophile
- Different ligands can form different numbers of dative bonds to the central metal ion in a complex
- Some ligands can form one dative bond to the central metal ion
- Other ligands can form two dative bonds, and some can form multiple dative bonds
- Co-ordination number is number of co-ordinate bonds to the central metal atom or ion
Common Ligands
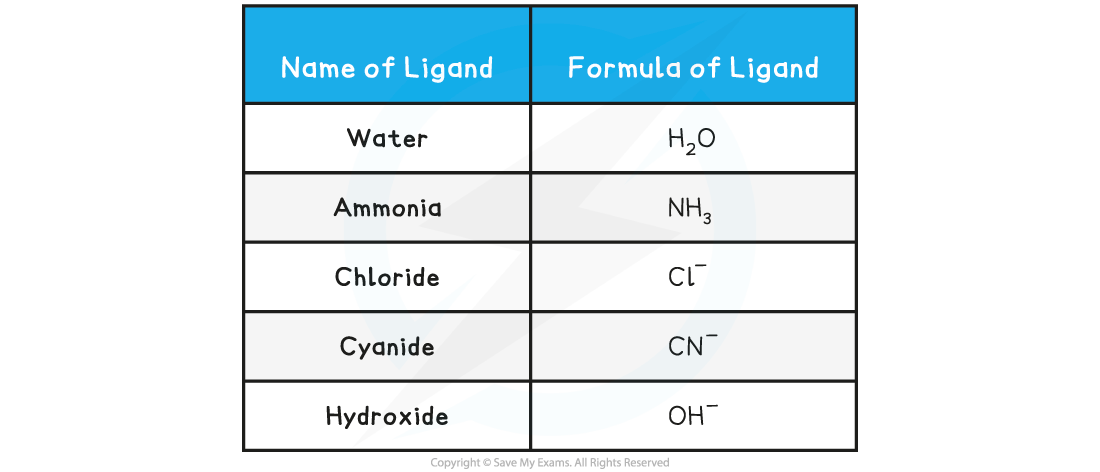
- Water molecules frequently act as ligands. Each water molecule makes a single bond with the metal ion using one of the lone pairs on the oxygen atom
- The lone pair is donated to the partially filled d-subshell of the transition metal ion
Table showing Examples of Common Monodentate Ligands
Representing complex ions
- Square brackets are used to group together the ligands and metal ion in a representation of the geometrical arrangement
- The overall charge on the complex ion is the sum of the oxidation states of all the species present
- If the ligands are neutral then the overall charge will be the same as the oxidation state of the metal ion
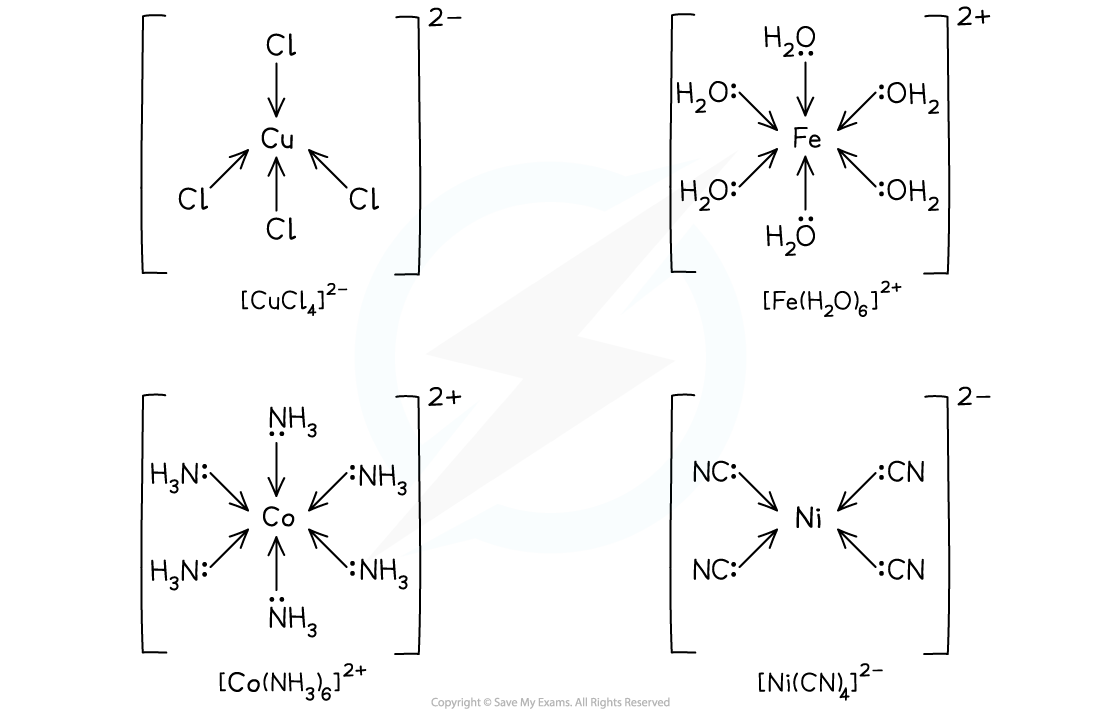
Examples of complexes with monodentate ligands
Naming complexes
- Complexes are named in the following way
- If the overall ion is a cation then the nomenclature is:
Prefix for number of ligands/ligand name/element/oxidation number
- The prefixes are the same ones used in organic chemistry: di, tetra, hexa for 2, 4 & 6 respectively (3 & 5 are rarely encountered except in mixed ligand complexes)
- If the overall ion is an anion, the name of element is modified to have the name ending 'ate' and sometimes Latin word stems are used
-
- tetrachlorcuprate(II)
- hexaaquairon(II)
- hexaamminecobalt(II)
- tetracyanonickelate(II)Using the examples in the illustration above, the names are:
- Notice in these examples that
- cuprate( Latin - cuprum) and nickelate are used in place of copper and nickel as they are anions
- Ammonia takes the prefix ammine as a ligand, which is spelt with a double 'm' unlike the functional group amine
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





