- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记6.1.2 Standard Electrode Potential
Standard Electrode Potentials
Standard electrode potential
- The position of equilibrium and therefore the electrode potential depends on factors such as:
- Temperature
- Pressure of gases
- Concentration of reagents
- So, to be able to compare the electrode potentials of different species, they all have to be measured against a common reference or standard
- Standard conditions also have to be used when comparing electrode potentials
- These standard conditions are:
- Ion concentration of 1.00 mol dm-3
- A temperature of 298 K
- A pressure of 100 kPa
- Standard measurements are made using a high resistance voltmeter so that no current flows and the maximum potential difference is achieved
- The electrode potentials are measured relative to a standard hydrogen electrode
- The standard hydrogen electrode is given a value of 0.00 V, and all other electrode potentials are compared to this standard
- This means that the electrode potentials are always referred to as a standard electrode potential (Eꝋ)
- The standard electrode potential (Eꝋ) is the potential difference ( sometimes called voltage) produced when a standard half-cell is connected to a standard hydrogen cell under standard conditions
- For example, the standard electrode potential of bromine suggests that relative to the hydrogen half-cell it is more likely to get reduced, as it has a more positive Eꝋ value
Br2(l) + 2e– ⇌ 2Br–(aq) Eꝋ = +1.09 V
2H+(aq) + 2e– ⇌ H2(g) Eꝋ = 0.00 V
- The standard electrode potential of sodium, on the other hand, suggests that relative to the hydrogen half-cell it is less likely to get reduced as it has a more negative Eꝋ value
Na+ (aq) + e– ⇌ Na(s) Eꝋ = -2.71 V
2H+ (aq) + 2e– ⇌ H2(g) Eꝋ = 0.00 V
The Standard Hydrogen Electrode, SHE
- The standard hydrogen electrode is a half-cell used as a reference electrode and consists of:
- Hydrogen gas in equilibrium with H+ ions of concentration 1.00 mol dm-3 (at 100 kPa)
2H+ (aq) + 2e- ⇌ H2 (g)
-
- An inert platinum electrode that is in contact with the hydrogen gas and H+ ions
- When the standard hydrogen electrode is connected to another half-cell, the standard electrode potential of that half-cell can be read off a high resistance voltmeter
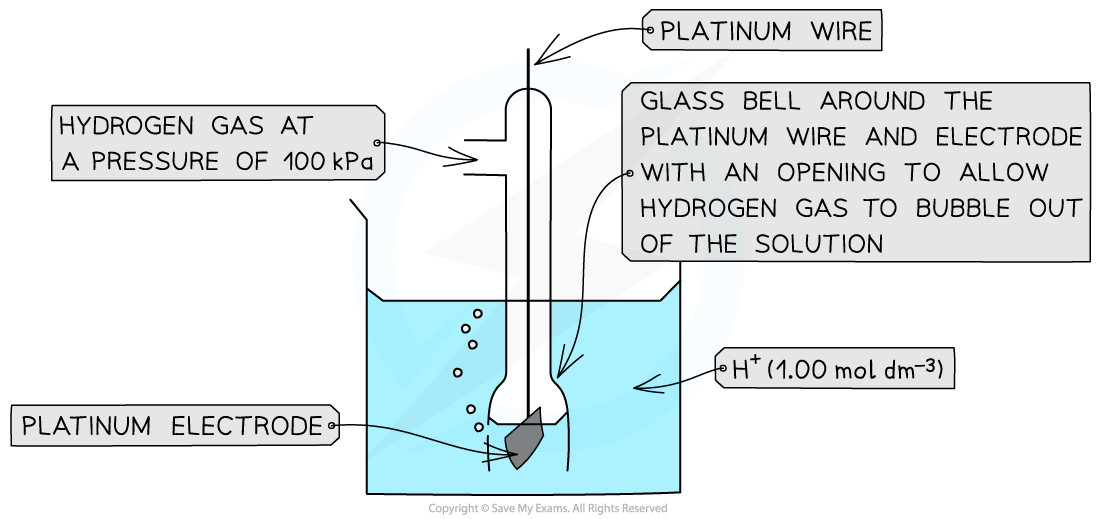
The standard electrode potential of a half-cell can be determined by connecting it to a standard hydrogen electrode
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





