- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记6.1.1 Reduction & Oxidation
Reduction & Oxidation
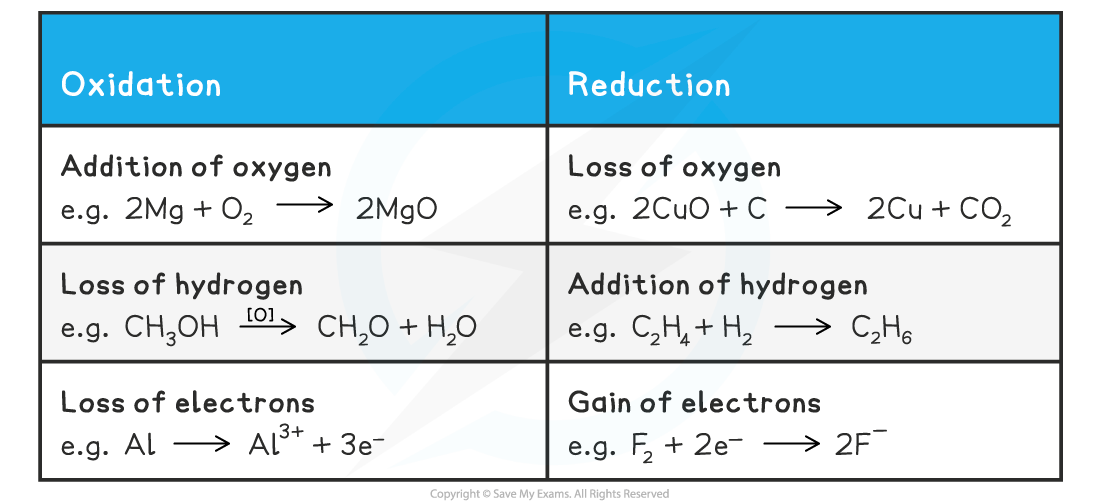
- To recap, there are three definitions of oxidation and reduction used in different branches of chemistry
- Oxidation and reduction can be used to describe any of the following processes
Definitions and Examples of Oxidation & Reduction
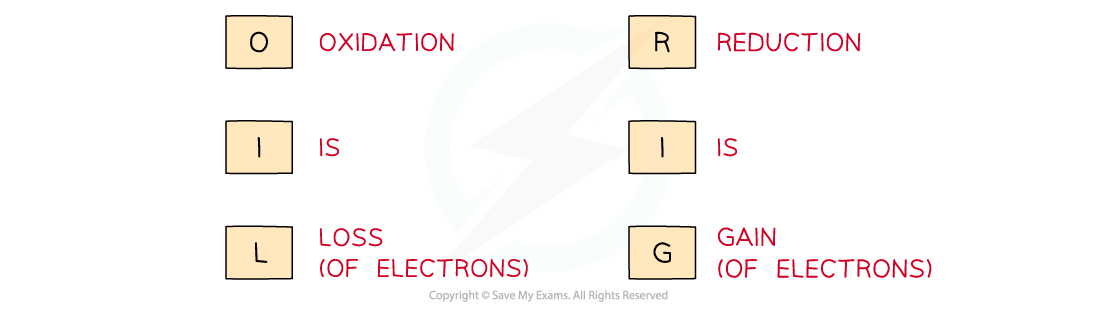
Use the acronym "Oil Rig" to help you remember the definitions of oxidation and reduction
- s-block elements are usually oxidised by losing electrons to form 1+ and 2+ ions
Na → Na+ + e–
Ca → Ca2+ + 2e–
- p-block metals typically undergo oxidation, by losing electrons, to form positive ions
- p-block metal ions can have a charge that is consistent with their group of the Periodic Table
Al → Al3+ + 3e–
-
- p-block metal ions can also form common ions with a charge that is not consistent with their group
Sn → Sn2+ + 2e–
- p-block non-metals are usually reduced, by gaining electrons, to form negative ions
- p-block non-metals form ions with a charge that can be calculated by (the group number - 8)
F + e- → F– (group 7 - 8 = -1)
O + 2e– → O2- (group 6 - 8 = -2)
- d-block elements can form various ions due to their variable oxidation states
- d-block elements will usually be oxidised, by losing electrons, to form positive ions, e.g. Cu2+, Cr3+, V5+
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





