- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记5.5.9 Arrhenius & Activation Energy
Arrhenius & Activation Energy
- The rate equation shows how each of the reactants in a reaction effects the rate of the reaction and it includes the rate constant, k
- However, k only remains constant if the concentration of the reactants is the only factor which is changed
- If the temperature is changed or a catalyst is used or changed, then the rate constant, k, changes
- At higher temperatures, a greater proportion of molecules have energy greater than than the activation energy
- Since the rate constant and rate of reaction are directly proportional to the fraction of molecules with energy equal or greater than the activation energy, then at higher temperatures:
- The rate of reaction increases
- The rate constant increases
- The relationship between the rate constant, the temperature and also the activation energy is given by the Arrhenius equation:

-
- Ea and A are constants that are characteristic of a specific reaction
- A does vary slightly with temperature but it can still be considered a constant
- R is a fundamental physical constant for all reactions
- k and T are the only variables in the Arrhenius equation
- Ea and A are constants that are characteristic of a specific reaction
- The Arrhenius equation is used to describe reactions that involve gases, reactions occurring in solution or reactions that occur on the surface of a catalyst
Finding the Activation Energy
- Very often, the Arrhenius equation is used to calculate the activation energy of a reaction
- A question will either give sufficient information for the Arrhenius equation to be used or a graph can be plotted and the calculation done from the plot
Using the Arrhenius Equation
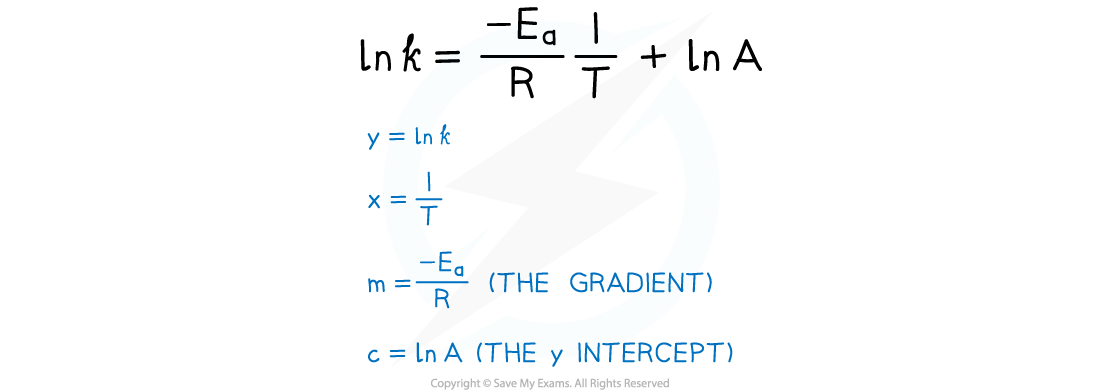
- The Arrhenius equation is easier to use if you take natural logarithms of each side of the equation, which results in the following equation:

- The Arrhenius Equation can be used to show the effect that a change in temperature has on the rate constant, k, and thus on the overall rate of the reaction
- An increase in temperature (higher value of T) gives a greater value of ln k and therefore a higher value of k
- Since the rate of the reaction depends on the rate constant, k, an increase in k also means an increased rate of reaction
- The equation can also be used to show the effect of increasing the activation energy on the value of the rate constant, k
- An increase in the activation energy, Ea, means that the proportion of molecules which possess at least the activation energy is less
- This means that the rate of the reaction, and therefore the value of k, will decrease
- The values of k and T for a reaction can be determined experimentally
- These values of k and T can then be used to calculate the activation energy for a reaction
- This is the most common type of calculation you will be asked to do on this topic
Worked Example
Calculate the activation energy of a reaction which takes place at 400 K, where the rate constant of the reaction is 6.25 x 10-4 s-1.
A = 4.6 x 1013 and R = 8.31 J mol-1 K-1.
Answer
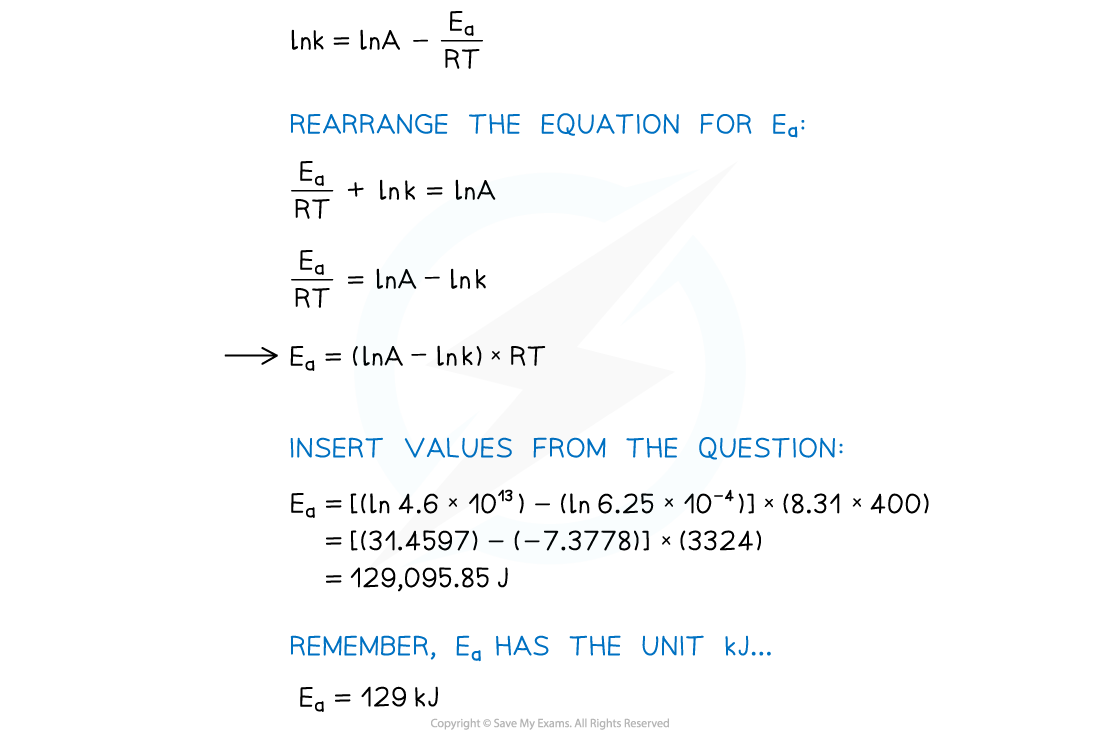
Using an Arrhenius plot:
- A graph of ln k against 1/T can be plotted, and then used to calculate Ea
- This gives a line which follows the form y = mx + c
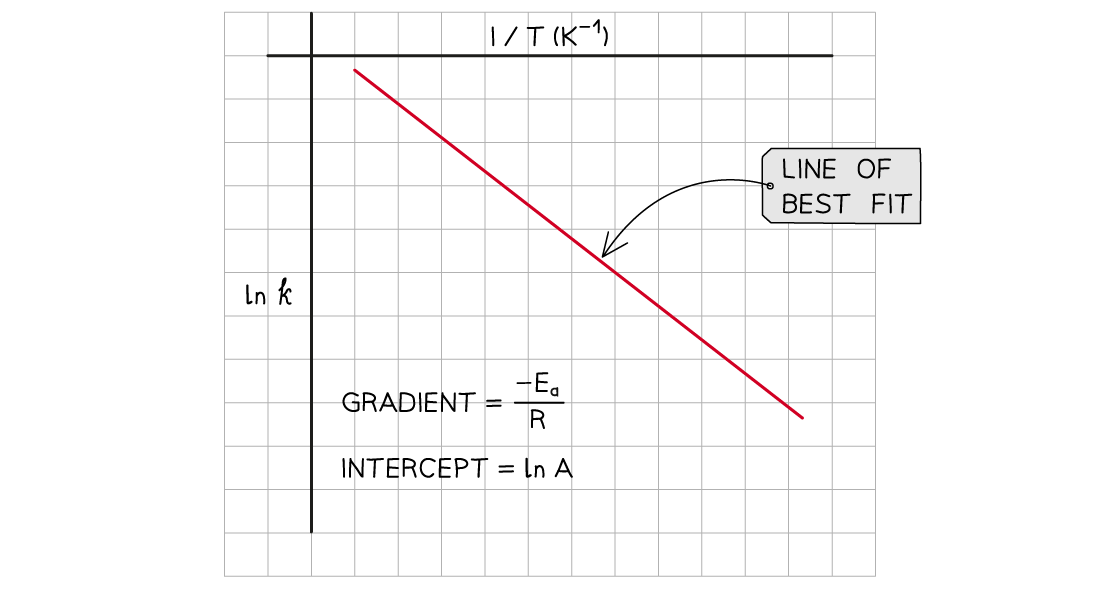
The graph of ln k against 1/T is a straight line with gradient -Ea/R
- From the graph, the equation in the form of y = mx + c is as follows:
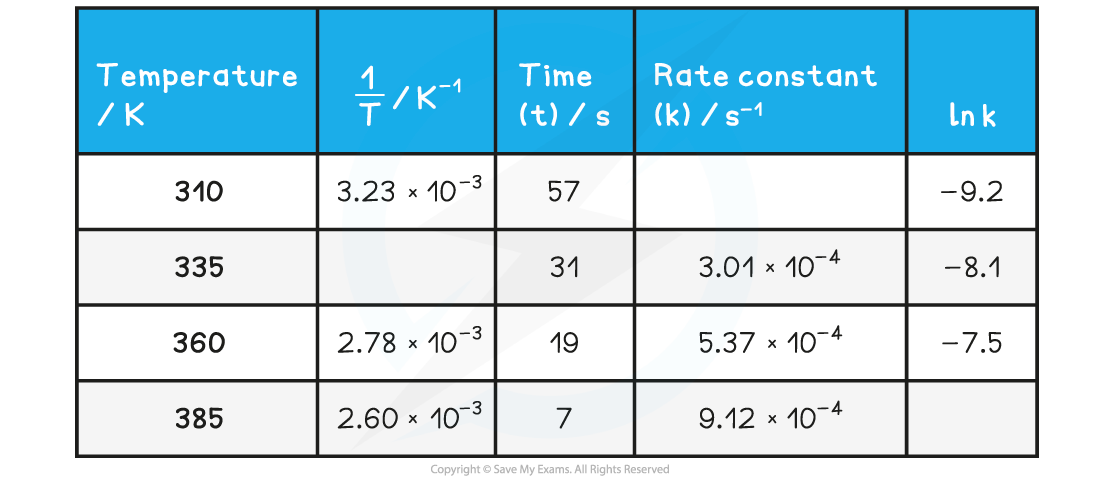
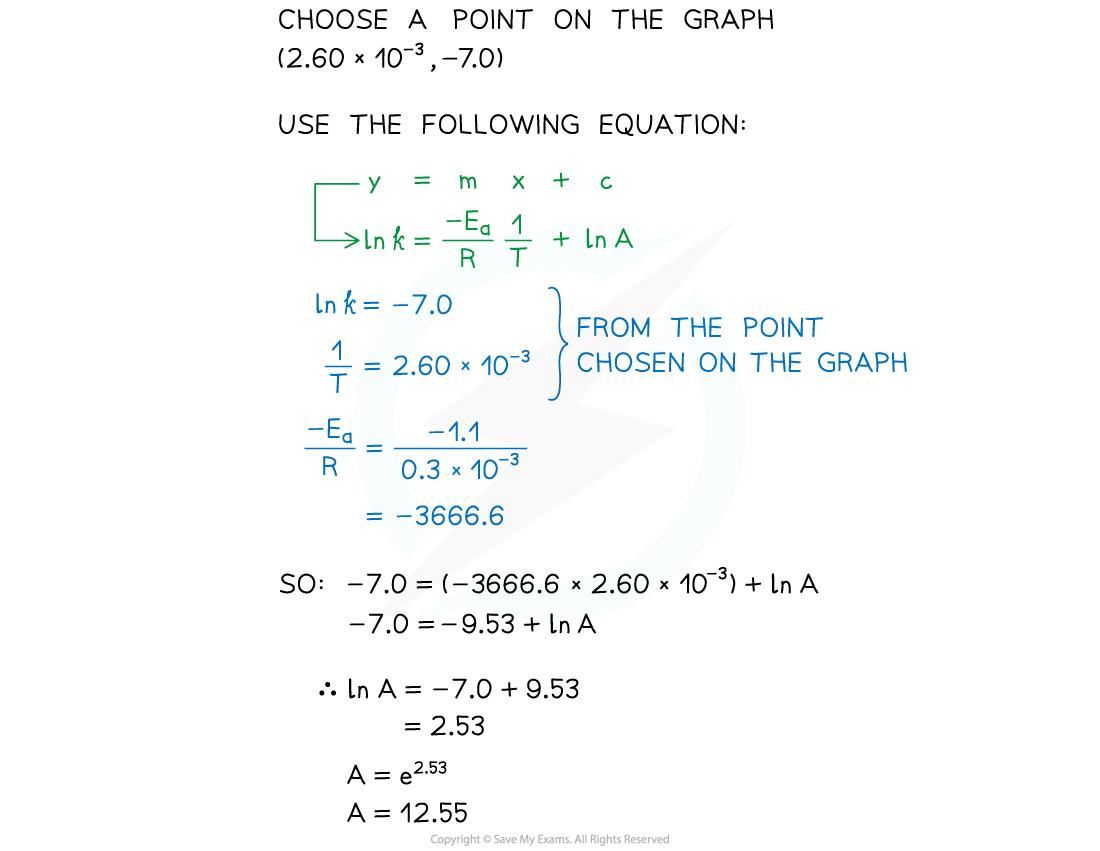
Worked Example
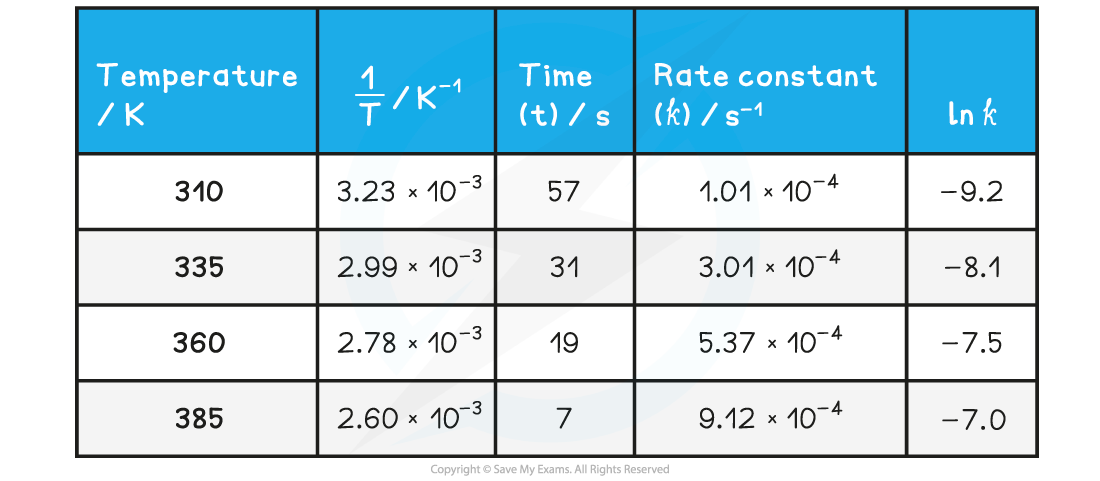
- Complete the following table
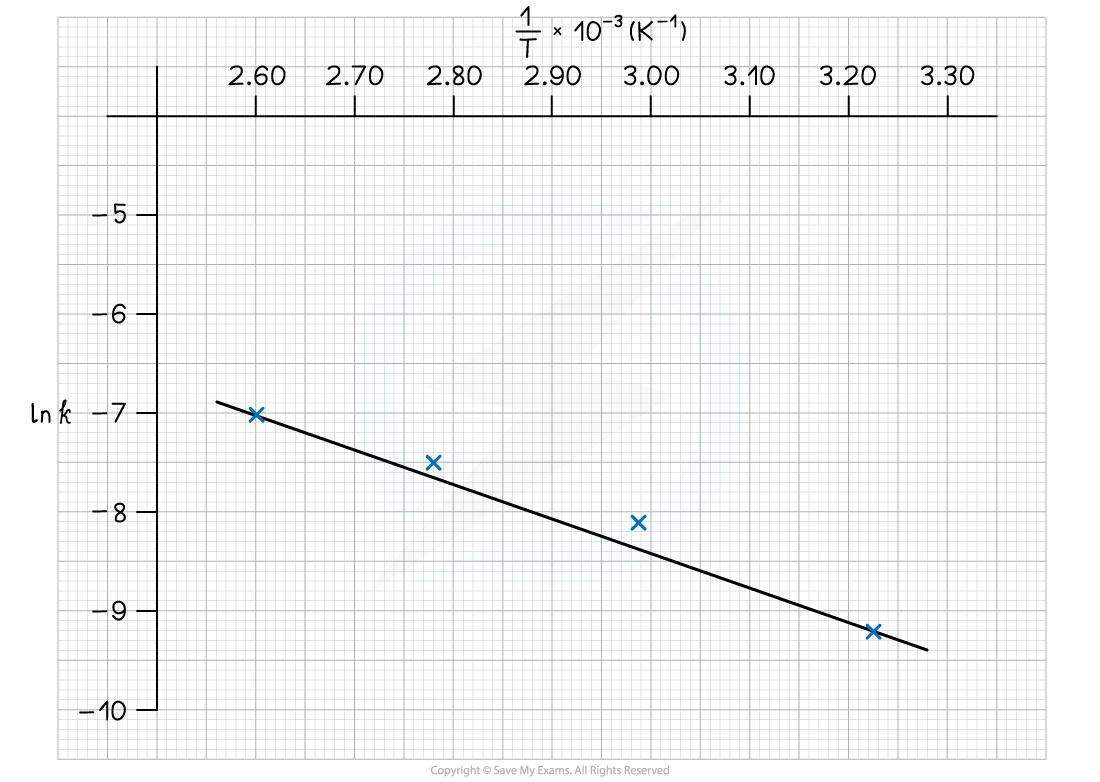
- Plot a graph of ln k against 1/T
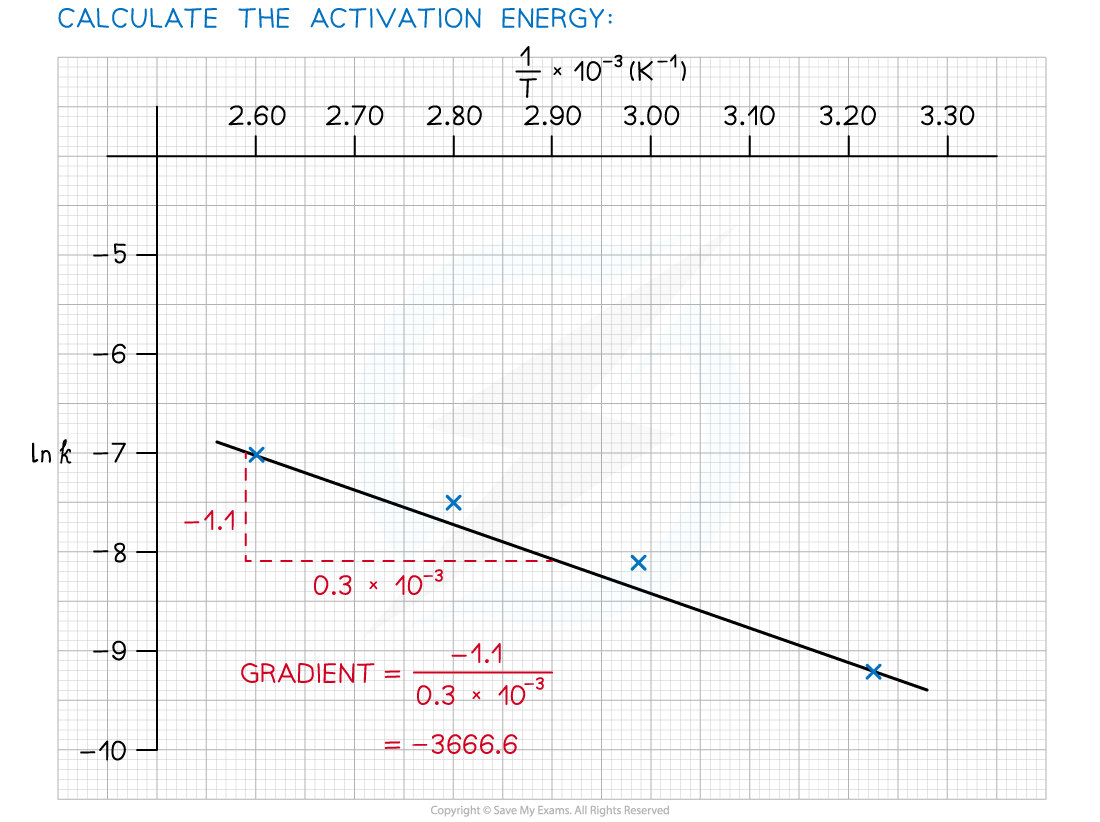
- Use this to calculate the activation energy, Ea, and the Arrhenius constant, A, of the reaction.
Answer 1:
Answer 2:
Answer 3:

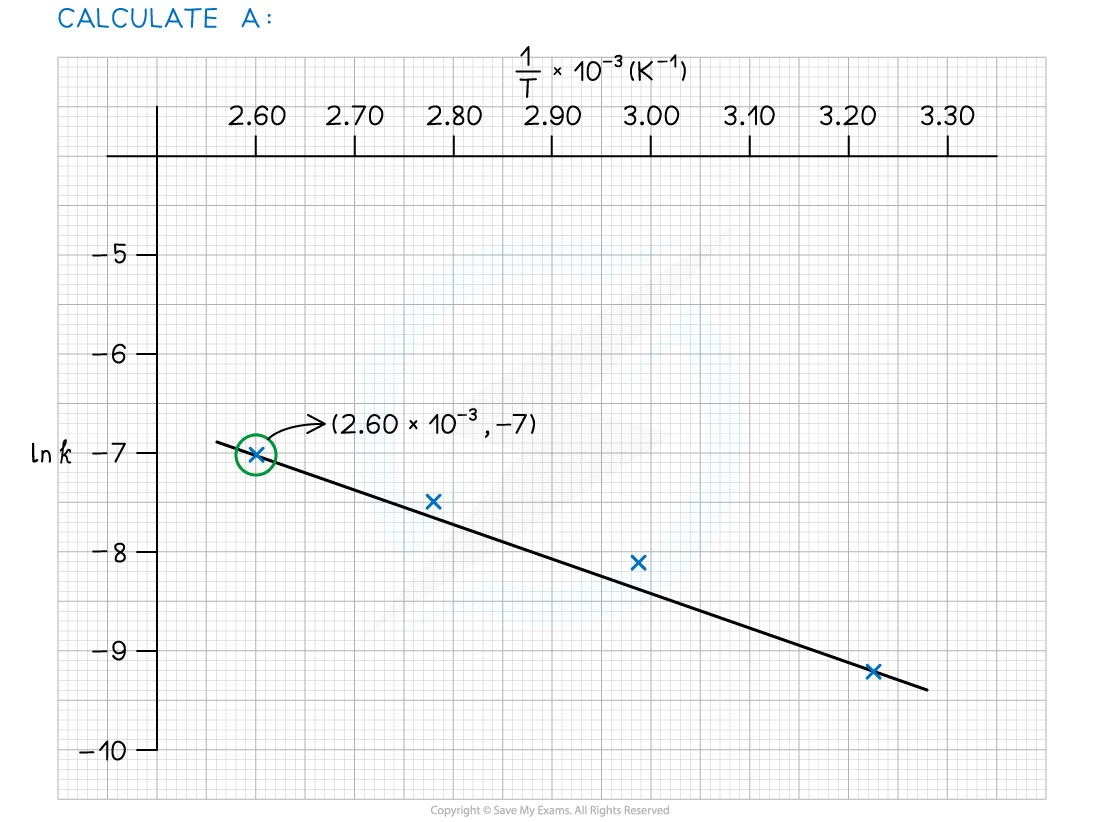
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





