- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记5.4.3 Gibbs Free Energy
Feasibility of Reactions
Gibbs free energy
- The feasibility of a reaction is determined by two factors
- The enthalpy and entropy change
- The two factors come together in a fundamental thermodynamic concept called the Gibbs free energy (G)
- The Gibbs equation is:
ΔGꝋ = ΔHreactionꝋ – TΔSsystemꝋ
-
- The units of ΔGꝋ are in kJ mol–1
- The units of ΔHreactionꝋare in kJ mol–1
- The units of T are in K
- The units of ΔSsystemꝋ are in J K-1 mol–1(and must therefore be converted to kJ K–1 mol–1by dividing by 1000)
- For a reaction to be feasible, ΔGꝋ must be equal or less than zero
Gibbs Free Energy Calculations
Calculating ΔGΘ
- This can be done by using the Gibbs equation, using enthalpy change, ΔHꝋ, and entropy change, ΔSꝋ, values
Worked Example
ΔGꝋfrom ΔHꝋand ΔSꝋvalues
Calculate the free energy change for the following reaction:
2NaHCO3 (s) → Na2CO3 (s) + H2O (l) + CO2 (g)
- ΔHꝋ = +135 kJ mol-1
- ΔSꝋ = +344 J K-1 mol-1
Answer:
Step 1: Convert the entropy value in kilojoules
ΔSꝋ = +344 J K-1 mol-1 ÷ 1000 = +0.344 kJ K-1 mol-1
Step 2: Substitute the terms into the Gibbs Equation
ΔGꝋ= ΔHreactionꝋ– TΔSsystemꝋ
= +135 – (298 x 0.344)
= +32.49 kJ mol-1
The temperature is 298 K since standard values are quoted in the question
- Rearranging the Gibbs equation allows you to determine the temperature at which a non-spontaneous reaction become feasible
ΔGꝋ = ΔHreactionꝋ - TΔSsystemꝋ
- Remember, for a reaction to be feasible ΔGΘꝋmust be zero or negative
0 = ΔHꝋ - TΔSꝋ
ΔHꝋ = TΔSꝋ
T = ΔHꝋ ÷ ΔSꝋ
Worked Example
At what temperature will the reduction of aluminium oxide with carbon become spontaneous?
Al2O3(s) + 3C(s)→ 2Al(s) + 3CO(g)
ΔHꝋ = +1336 kJ mol-1
ΔSꝋ = +581 J K-1 mol-1
Answer:
-
- If ΔGꝋ = 0 then T = ΔHꝋ ÷ ΔSꝋ
- Covert ΔSꝋ to kJ K-1 mol-1 by dividing by 1000
- T = 1336 ÷ (581/1000)
- T = 2299 K
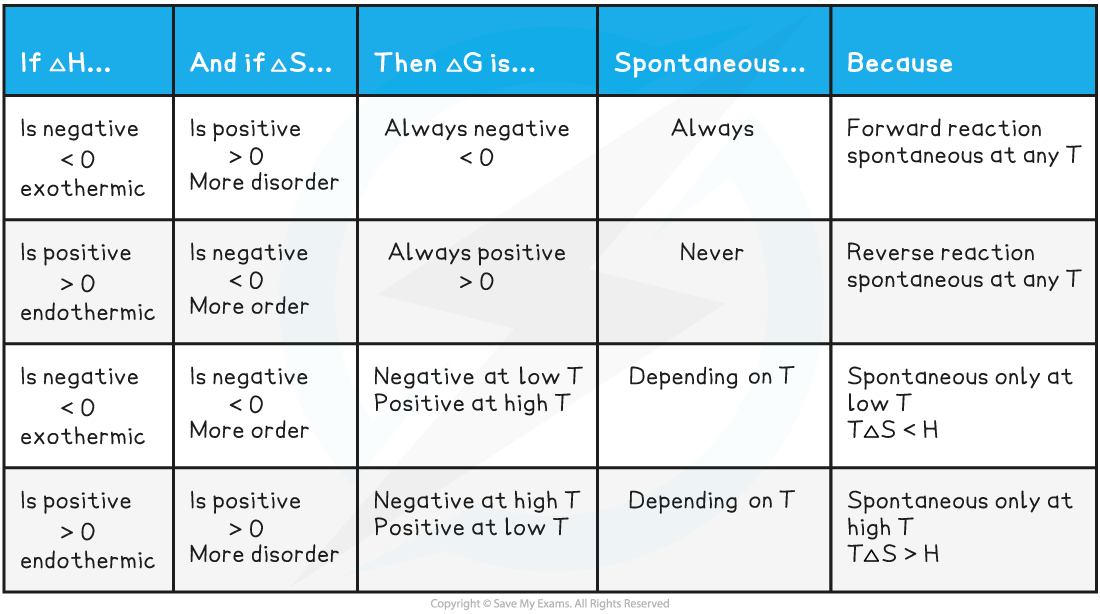
Temperature and Gibbs free energy
- When ΔGꝋ is negative, the reaction is spontaneous / feasible and likely to occur
- When ΔGꝋ is positive, the reaction is not spontaneous / feasible and unlikely to occur
- We can also look at the the values for enthalpy change, ΔH, and entropy change, ΔS
- Depending on the value for ΔH and ΔS we can determine whether the reaction is spontaneous at a given temperature (T)
Summary for temperature and Gibbs free energy
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





