- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记4.2.2 Ethanol Oxidation
Core Practical 5: Oxidation of Ethanol
Oxidation of ethanol
- Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids
- When ethanol is oxidised, ethanal is produced and when oxidised further ethanoic acid will be formed
Synthesis and purification of ethanal and ethanoic acid
- Carefully add 20 cm3 of acidified potassium dichromate(VI) solution, K2Cr2O7 (aq), to a 50 cm3 pear-shaped flask and cool the flask in an iced water bath
- Set up the reflux apparatus keeping pear shaped flask cool
- Place anti-bumping granules into the pear shaped flask
- Measure out 1 cm3 of ethanol
- Using a pipette, slowly add the ethanol drop wise into the reflux condenser
- When the ethanol has been added remove the ice bath and allow to warm up to room temperature
- Position the flask over an electric heater or in a water bath and heat for 20 minutes
- Ethanol is flammable, therefore. naked flames should not be used when heating which is why the use of an electric heater or water bath is an important safety precaution
- Purify the product using distillation apparatus
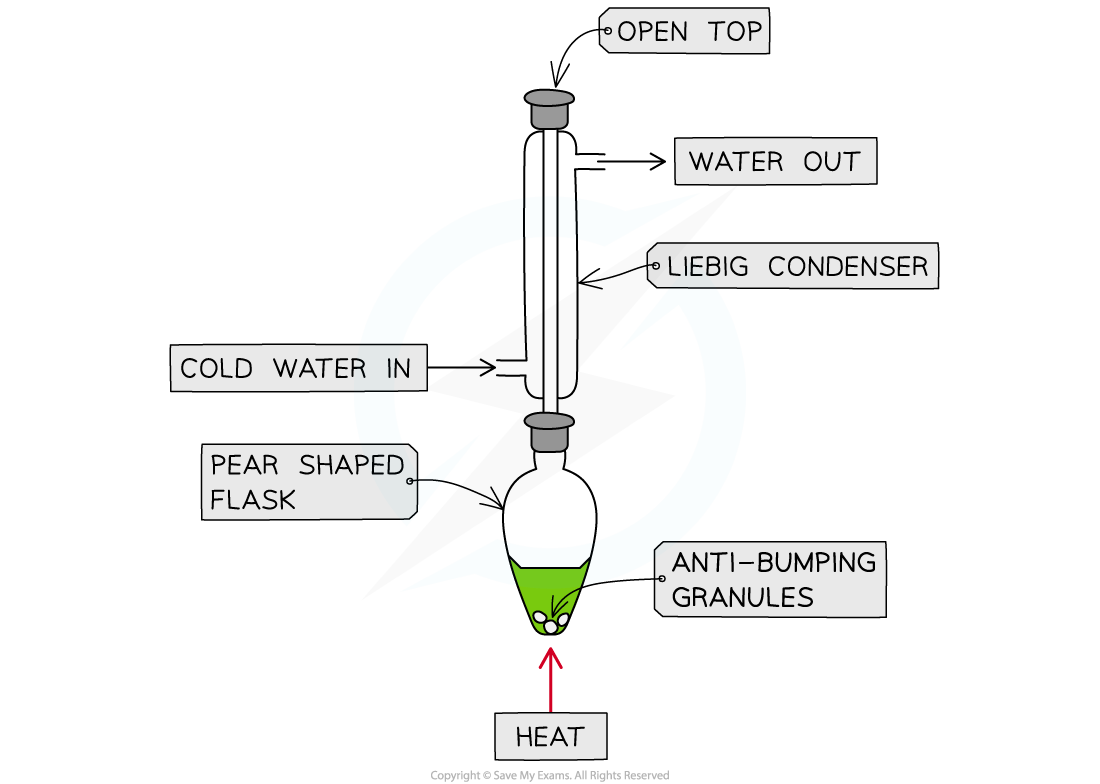
Reflux apparatus for the oxidation of ethanol to ethanoic acid
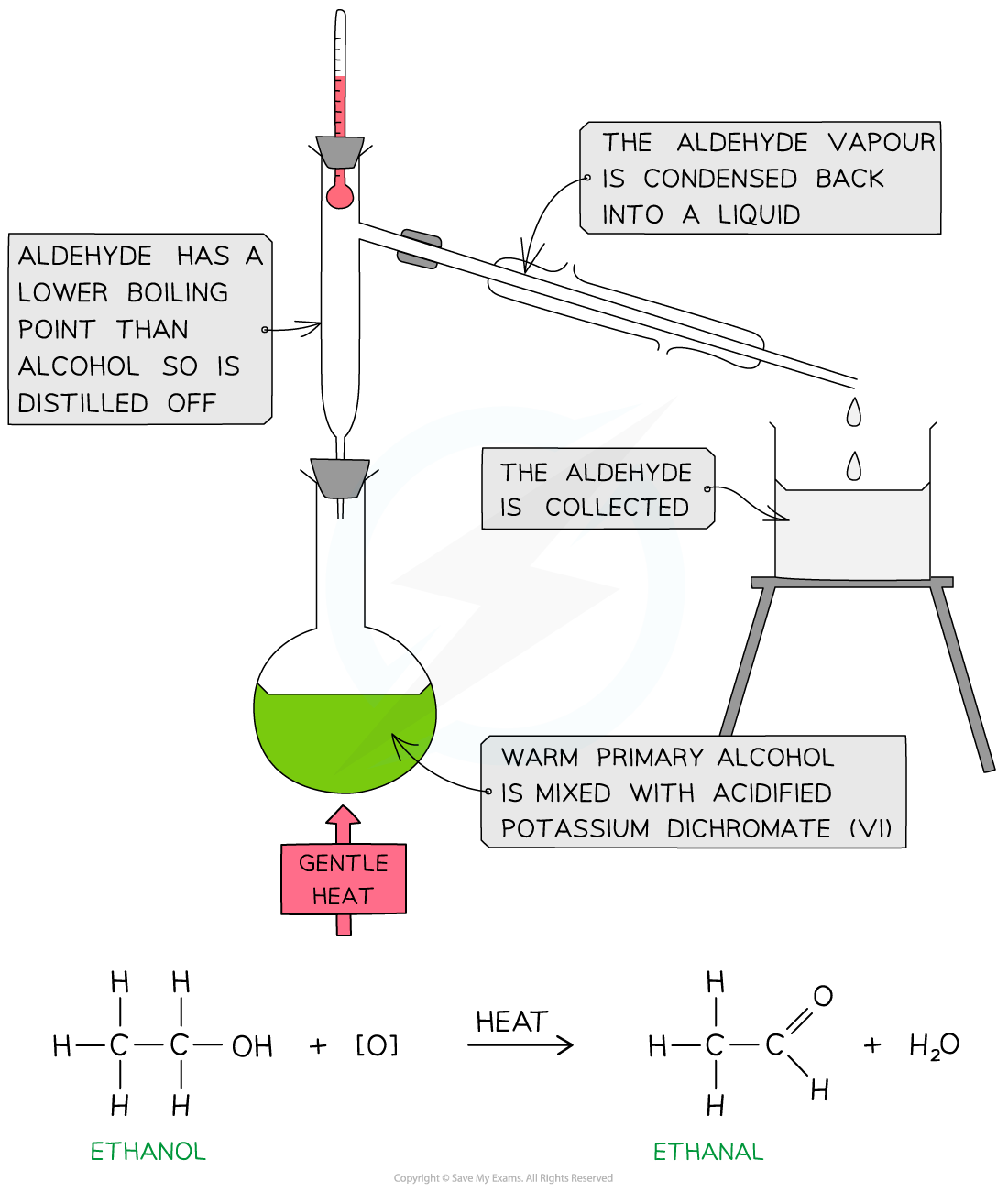
Oxidation of ethanol by acidified K2Cr2O7 to form an aldehyde by distillation
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





