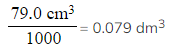- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记4.1.1 Molar Volume of a Gas
Core Practical 1: Measuring the Molar Volume of a Gas
Measuring gas volumes
- The volume of gas produced in a reaction can be measured by collecting the gas with a gas syringe or by the displacement of water
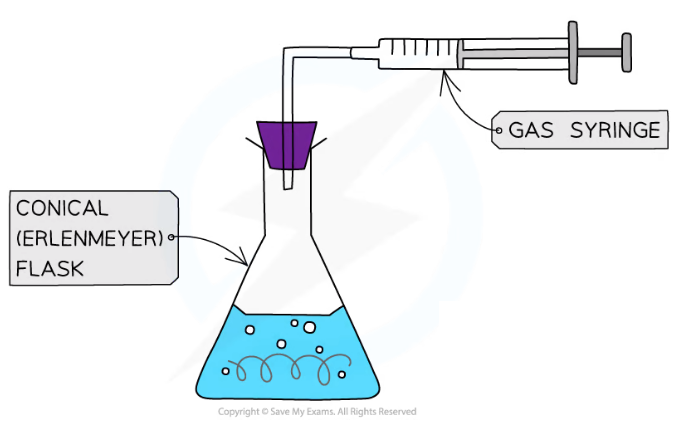
Gas syringe equipment for collecting the gas produced in a reaction
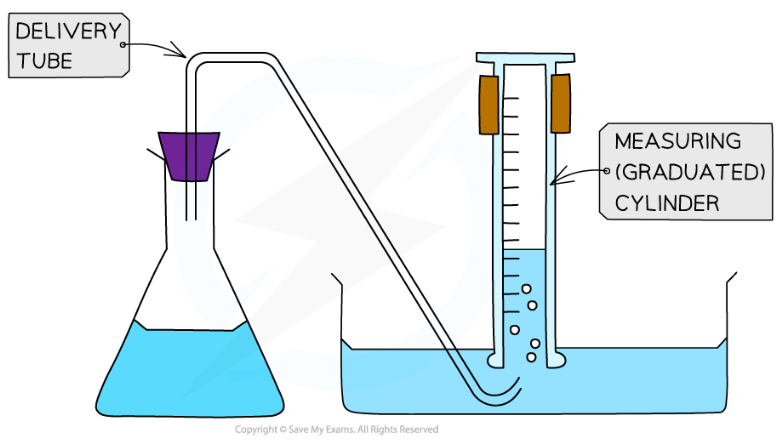
Displacement of water equipment for collecting the gas produced in a reaction
Sample method
- For the reaction of hydrochloric acid and sodium carbonate
Na2CO3 (s) + 2HCl (aq) → 2NaCl (aq) + H2O (l) + CO2 (g)
- Measure out a fixed volume of hydrochloric acid, e.g. 25.0 cm3, into a conical flask
- Add a known mass of sodium carbonate, e.g. 0.05 g, to the conical flask
- Immediately connect the gas syringe delivery tube
- Allow the reaction to go to completion
- Record the volume of carbon dioxide produced
- Repeat the experiment with different masses of sodium carbonate, e.g. 0.10 g, 0.15 g, 0.20 g, 0.25 g... 0.50 g
- Some assumptions are made about the experiment:
- The amount of gas lost between adding the sodium carbonate and connecting the delivery tube is negligible
- The delivery tube set up is airtight so no gas is lost
- The reaction does go to completion
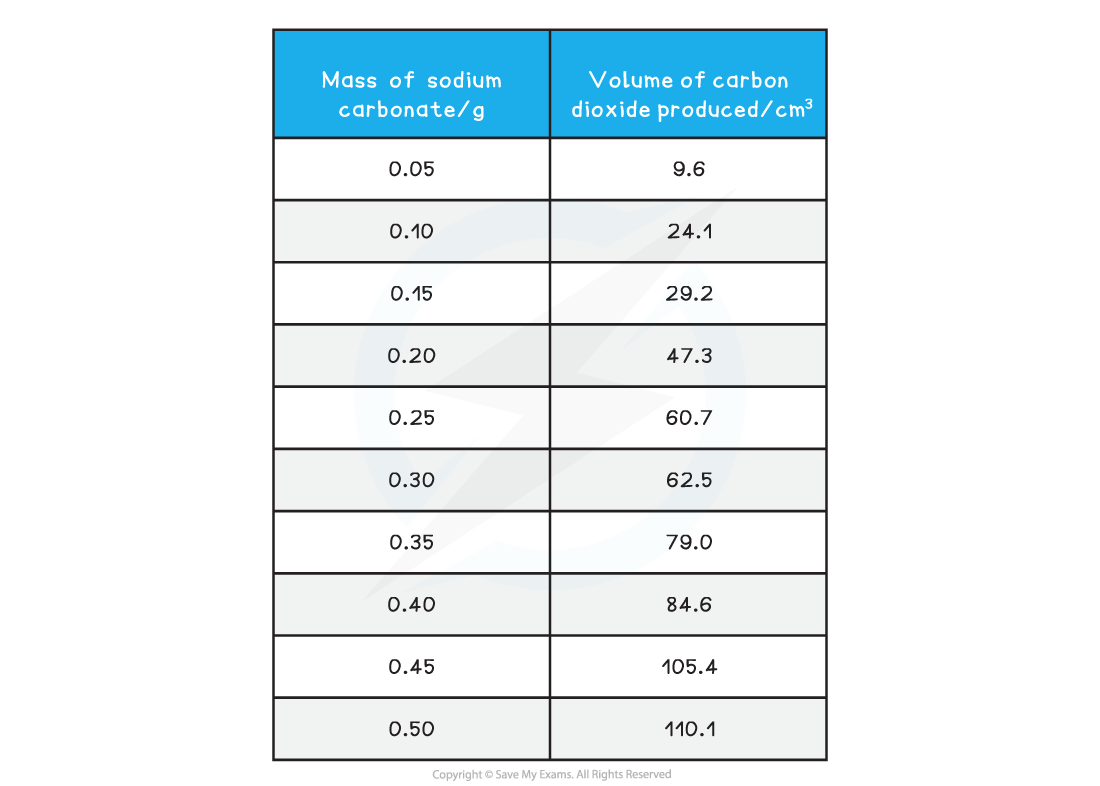
Sample results
Mass volume results table
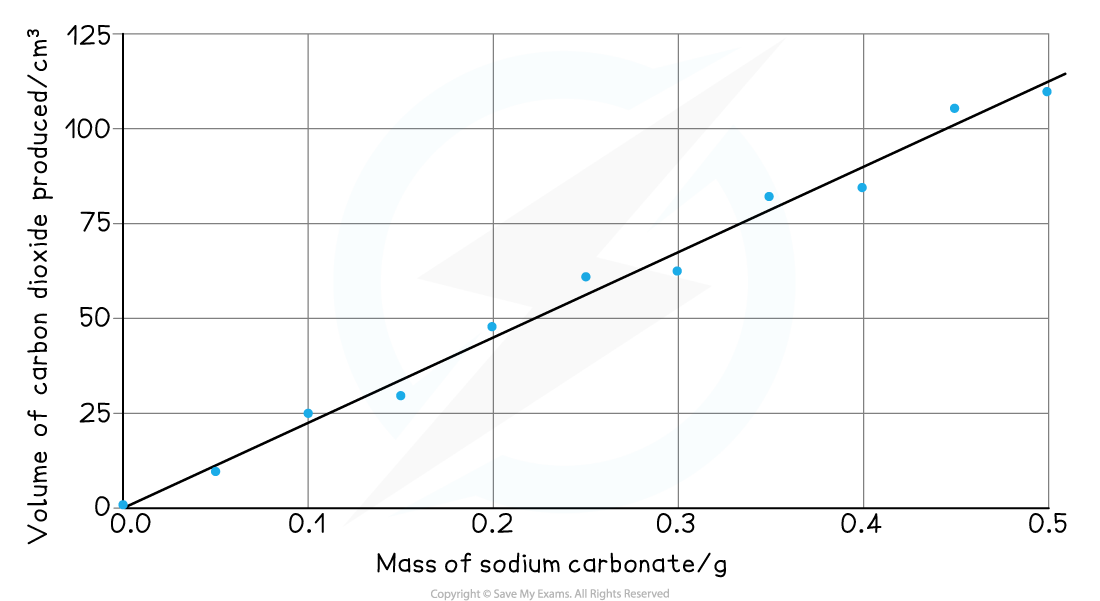
- The results are then plotted on to a graph
- Mass of sodium carbonate on the x-axis and volume or carbon dioxide produced on the y-axis
- Anomalous results are ignored and one straight line (or one smooth curve) of best fit is added
Analysis
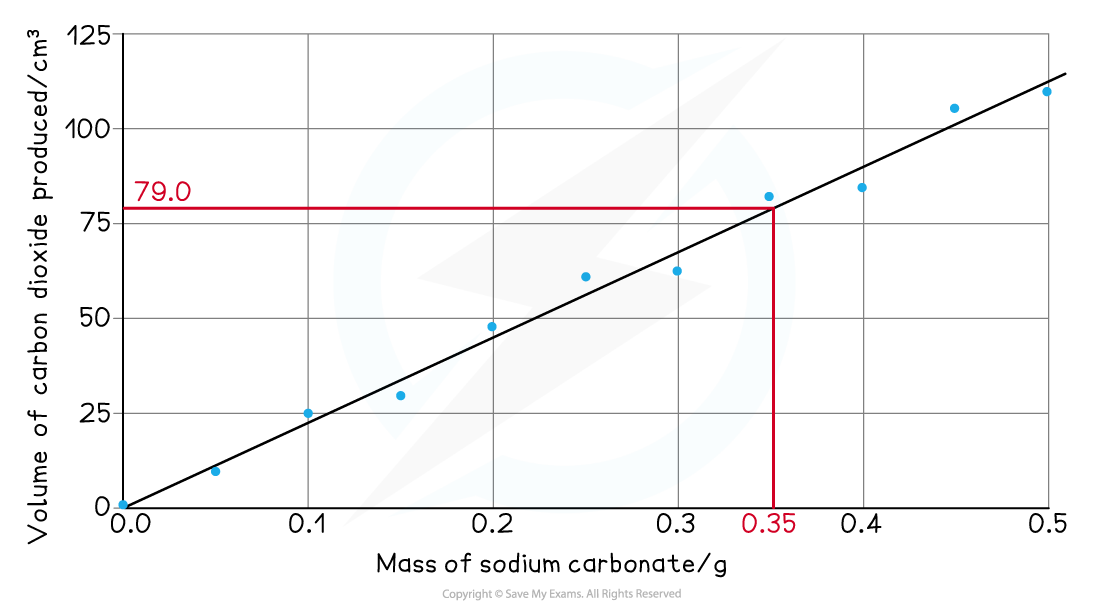
- Read off the volume of gas produced for a sensible mass of sodium carbonate, e.g. 0.35 g produces 79.0 cm3
- The mass of sodium carbonate may be specified in an exam question
Na2CO3 (s) + 2HCl (aq) → 2NaCl (aq) + H2O (l) + CO2 (g)
- From the reaction equation, one mole of sodium carbonate produces one mole of carbon dioxide
- Calculate the molar mass of sodium carbonate
- (2 x 23.0) + 12.0 + (3 x 16.0) = 106.0
- Calculate the number of moles of sodium carbonate, using the mass from your graph reading
- Moles
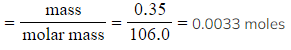
- Moles
- Convert the volume of carbon dioxide from your graph reading from cm3 to dm3
- Calculate the molar volume of gas produced:
- Molar gas volume
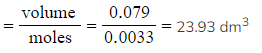
- Molar gas volume
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1