- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记3.5.1 Classifying Alcohols
Classifying Alcohols
- Alcohols are a family of molecules that contain the hydroxyl functional group, -OH
- Their general formula is CnH2n+1OH
- The nomenclature of alcohols follows the pattern alkan + ol
- If there are two -OH groups present the molecule is called a diol
The first four Alcohols and their Structures Table
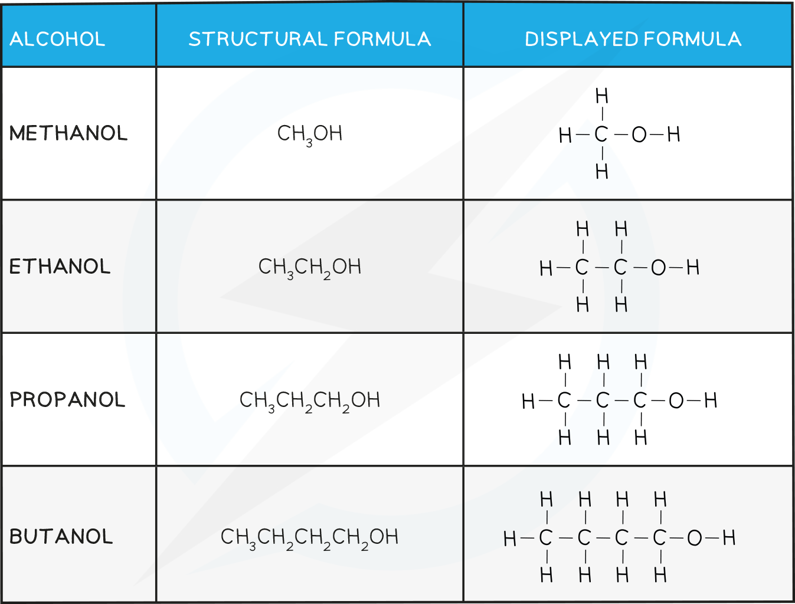
- Alcohols are classified as primary, secondary or tertiary depending on the number of carbons attached to the functional group carbon
- Primary alcohols are alcohols in which the carbon atom bonded to the -OH group is attached to one other carbon atom (or alkyl group)
- Secondary alcohols are alcohols in which the carbon atom bonded to the -OH group is attached to two other carbon atoms (or alkyl groups)
- Tertiary alcohols are alcohols in which the carbon atom bonded to the -OH group is attached to three other carbon atoms (or alkyl groups)
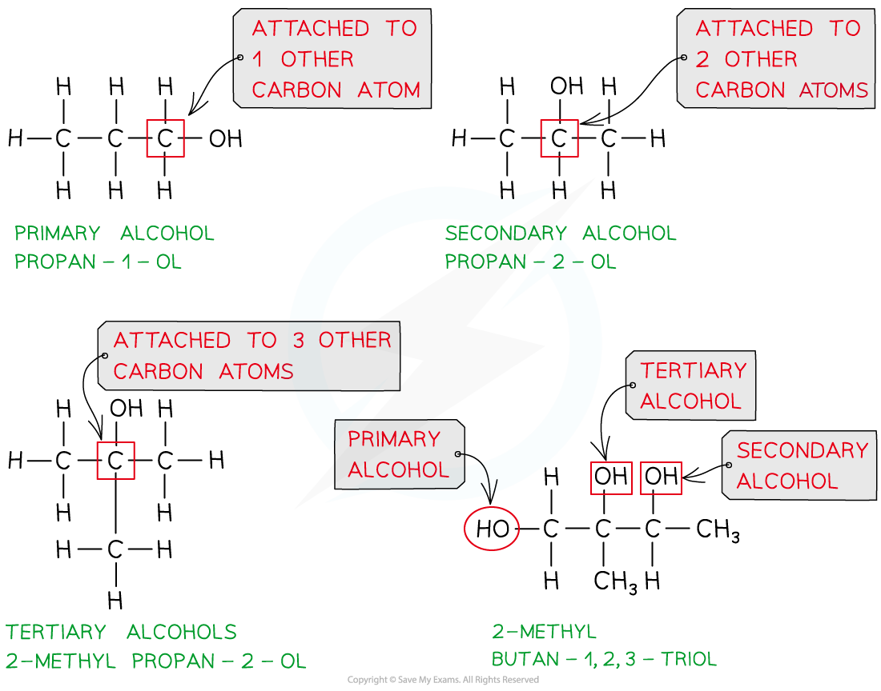
Classifying primary, secondary and tertiary alcohols and alcohols with more than one alcohol group
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





