- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记3.4.4 Hydrolysis of Halogenoalkanes
Relative Rates of Hydrolysis
Measuring the rate of hydrolysis
- Acidified aqueous silver nitrate can be used to measure the rate of hydrolysis of halogenoalkanes
- The following method is used:
- Set up three test tubes in a 50 oC water bath, with a mixture of ethanol and acidified silver nitrate
- Add a few drops of a chloroalkane, bromoalkane and an iodoalkane to each test tube and start a stop watch
- Time how long it takes for the precipitates to form
- Reacting halogenoalkanes with aqueous silver nitrate solution will result in the formation of a precipitate
- The rate of formation of these precipitates can also be used to determine the reactivity of the haloalkane
Haloalkane Precipitates Table
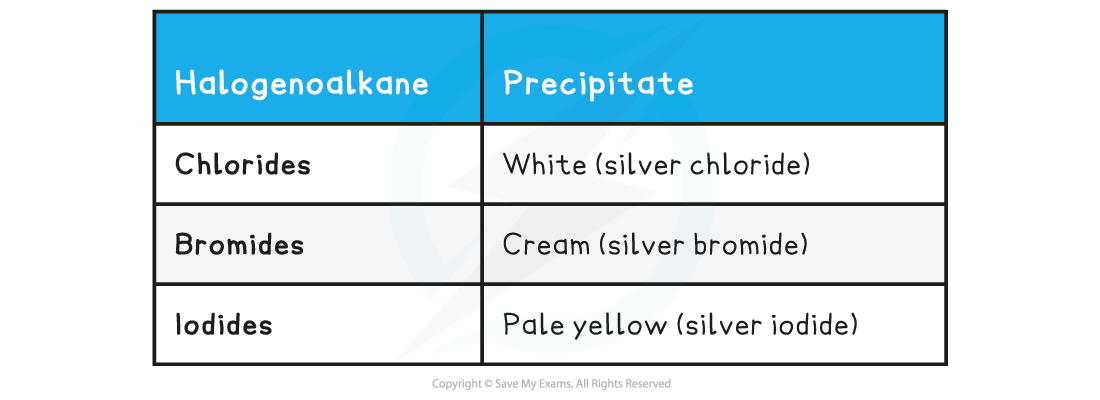
- The precipitates will form as the reaction progresses and the halide ions are formed
- The yellow silver iodide precipitate is the fastest nucleophilic substitution reaction
- This is because the C-I bond has the lowest bond enthalpy, so it is the easiest to break and will cause the I- ions to form the fastest
- The white chloride precipitate is the slowest nucleophilic substitution reaction
- This is because the C-Cl bond has the highest bond enthalpy, so it is the hardest to break and will cause the Cl- ions to form the slowest
- The yellow silver iodide precipitate is the fastest nucleophilic substitution reaction
-
- Silver fluoride is soluble, so a precipitate will not be formed in this reaction
- This confirms that fluoroalkanes are the least reactive and iodoalkanes are the most reactive halogenoalkanes
- It can be predicted that the formation of silver astatide would be even quicker than silver iodide

The trend in reactivity of haloalkanes
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





