- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记3.4.2 Nucleophilic Substitution
Nucleophilic Substitution: Reactions
- A nucleophile is an electron-rich species that can donate a pair of electrons
- ‘Nucleophile’ means ‘nucleus / positive charge loving’ as nucleophiles are attracted to positively charged species
- Nucleophilic refers to reactions that involve a nucleophile
Formation of alcohols
- The nucleophile in this reaction is the hydroxide ion, OH-
- An aqueous solution of sodium hydroxide (NaOH) or potassium hydroxide (KOH) with ethanol is used to form an alcohol
- This reaction is very slow at room temperature, so the reaction mixture is warmed
- This is an example of a hydrolysis reaction and the product is an alcohol
- The rate of this reaction depends on the type of halogen in the halogenoalkane
- The stronger the C-X bond, the slower the rate of the reaction
- In terms of bond enthalpy, C-F > C-Cl > C-Br > C-I
- Fluoroalkanes do not react at all, but iodoalkanes have a very fast rate of reaction

The halogen is replaced by the nucleophile, OH-
- This reaction could also be done with water as the nucleophile, but it is very slow
- The hydroxide ion is a better nucleophile than water as it carries a full negative charge
- In water, the oxygen atom only carries a partial charge

A hydroxide ion is a better nucleophile as it has a full formal negative charge whereas the oxygen atom in water only carries a partial negative charge; this causes the nucleophilic substitution reaction with water to be much slower than the aqueous alkali
Reaction with water
- The water molecule is a weak nucleophile, but it will eventually substitute for the halogen
- This occurs much more slowly compared to when warm aqueous sodium hydroxide is used
- An alcohol is produced
- RX + H2O → ROH + H+ + X-
- CH3CH2Br + H2O → CH3CH2OH + H+ + Br-
- If silver nitrate solution in ethanol is added to the solution, the silver ions will react with the halide ions as soon as they form, giving a silver halide precipitate
- Ag+ (aq) + X- (aq) → AgX (s)
Formation of nitriles
- The nucleophile in this reaction is the cyanide ion, CN-
- An ethanolic solution of potassium cyanide (KCN in ethanol) is heated under reflux with the halogenoalkane
- The product is a nitrile
- E.g. bromoethane is heated under reflux with ethanolic potassium cyanide to form propanenitrile

The halogen is replaced by a cyanide group, CN -
- The nucleophilic substitution of halogenoalkanes with KCN adds an extends the carbon chain by adding an extra carbon atom
- This reaction can therefore be used by chemists to make a compound with one more carbon atom than the best available organic starting material
Formation of primary amines by reaction with ammonia
- The nucleophile in this reaction is the ammonia molecule, NH3
- An ethanolic solution of excess ammonia (NH3 in ethanol) is heated under pressure with a primary halogenoalkane
- An excess of ammonia is used because the product is more reactive than ammonia so further substitution reactions could occur
- The product is a primary amine
- E.g. bromoethane reacts with excess ethanolic ammonia when heated under pressure to form ethylamine

The halogen is replaced by an amine group, NH2
Formation of alkenes
- The halogenoalkanes are heated with ethanolic sodium hydroxide causing the C-X bond to break heterolytically, forming an X- ion and leaving an alkene as an organic product
- E.g. bromoethane is heated with ethanolic sodium hydroxide to form ethene
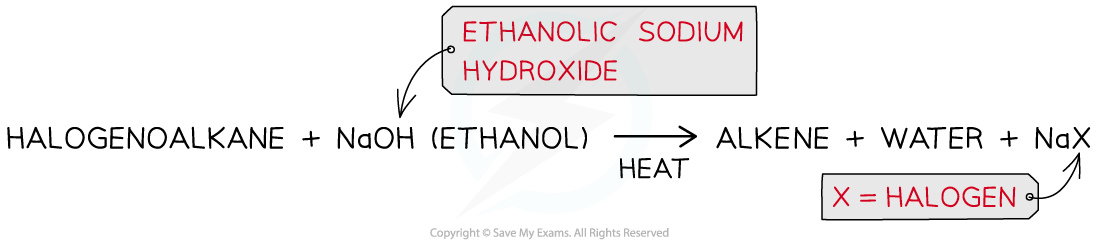
Production of an alkene from a halogenoalkane by reacting it with ethanolic sodium hydroxide and heating it
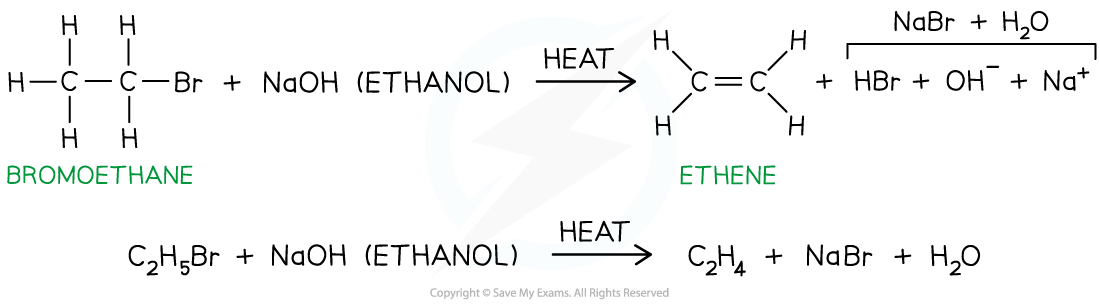
Hydrogen bromide is eliminated to form ethene
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





