- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记3.3.1 Describing Alkenes
Describing Alkenes
- All alkenes contain a double carbon bond, which is shown as two lines between two of the carbon atoms i.e. C=C
- All alkenes contain a double carbon bond, which is the functional group and is what allows alkenes to react in ways that alkanes cannot
- Alkenes have the general molecular formula CnH2n
- They are said to be unsaturated hydrocarbons
- They contain carbon-carbon double bonds
- They are made up of hydrogen and carbon atoms only
- Alkenes are named using the nomenclature rule alk + ene
- In molecules with a straight chain of 4 or more carbon atoms, the position of the C=C double bond must be specified
- The carbon atoms on the straight chain must be numbered, starting with the end closest to the double bond
- The lowest-numbered carbon atom participating in the double bond is indicated just before the -ene:
The First Five Members of the Alkene Family
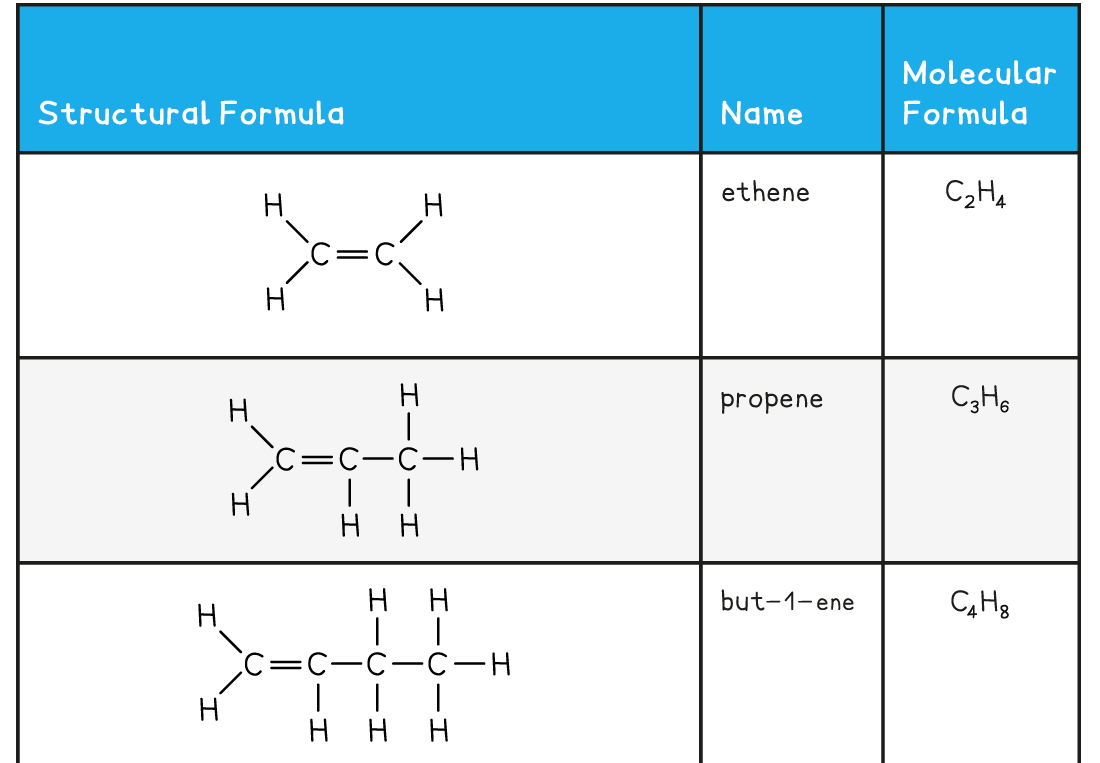
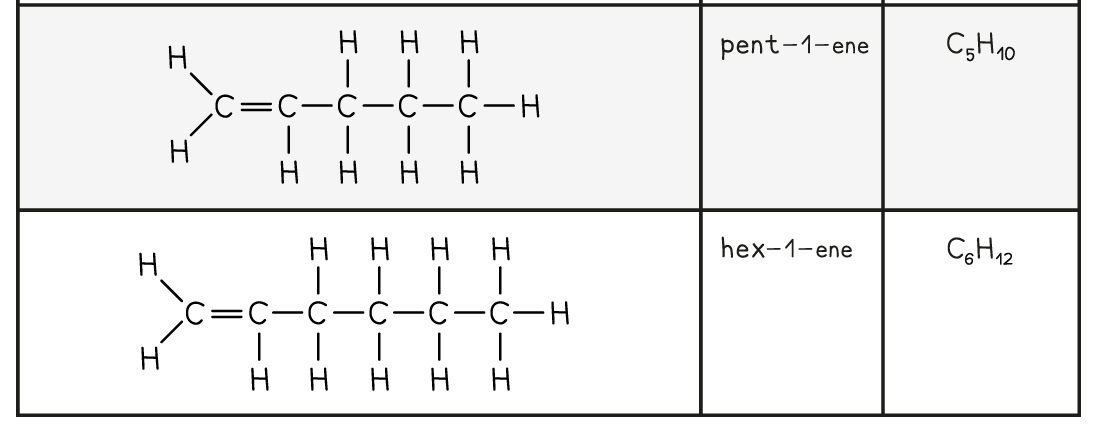
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





