- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记3.2.2 Alkane Fuels
Alkane Fuels
- Alkanes are obtained from the fractional distillation and cracking of crude oil
Fractional distillation of crude oil
- Crude oil is a mixture of hydrocarbons containing alkanes, cycloalkanes and arenes (compounds with a benzene ring)
- The crude oil is extracted from the earth in a drilling process and transported to an oil refinery
- At the oil refinery the crude oil is separated into useful fuels by fractional distillation
- This is a separating technique in which the wide range of different hydrocarbons are separated into fractions based on their boiling points
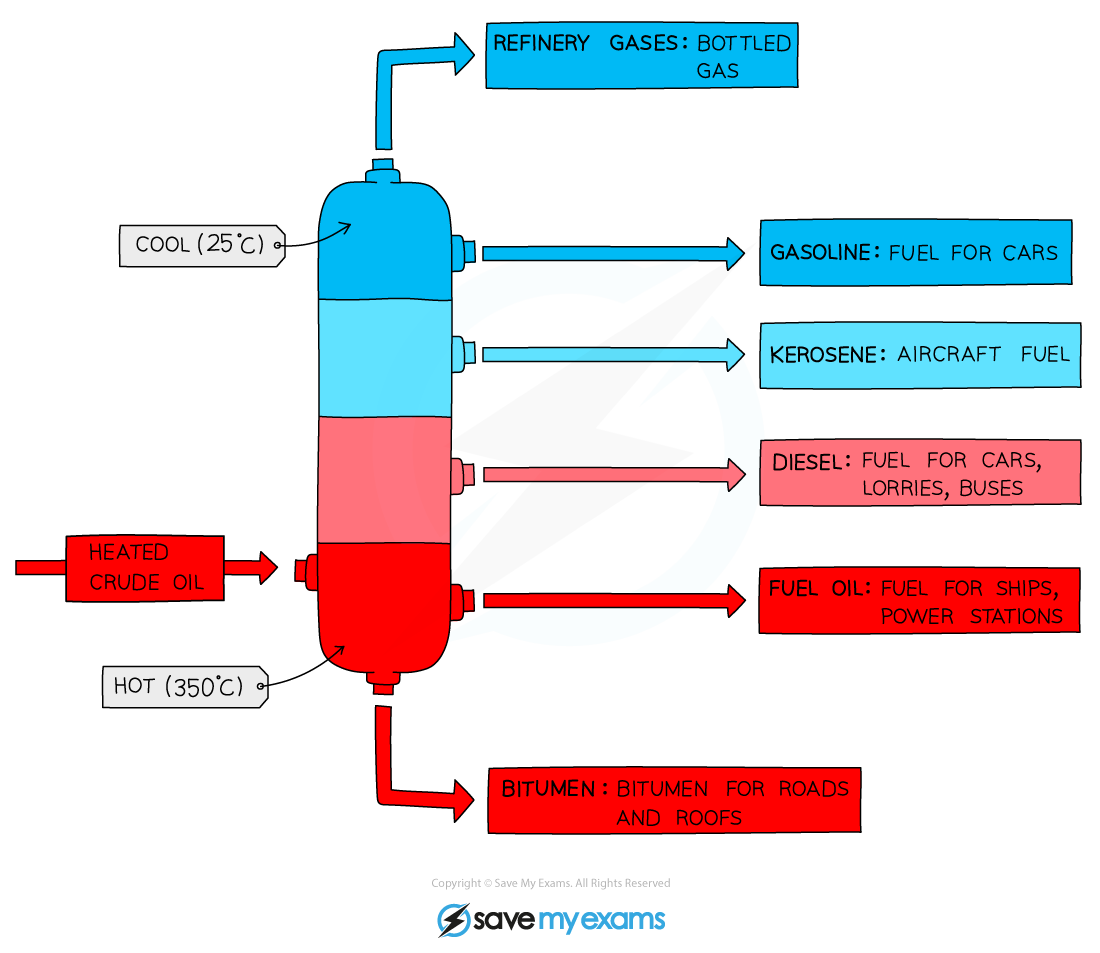
Crude oil is initially separated into fractions with similar boiling points in a process called fractional distillation
Cracking of crude oil fractions
- However, the smaller hydrocarbon fractions (such as gasoline fractions) are in high demand compared to the larger ones
- Therefore, some of the excess heavier fractions are broken down into smaller, more useful compounds
- These more useful compounds include alkanes and alkenes of lower relative formula mass (Mr)
- This process is called cracking
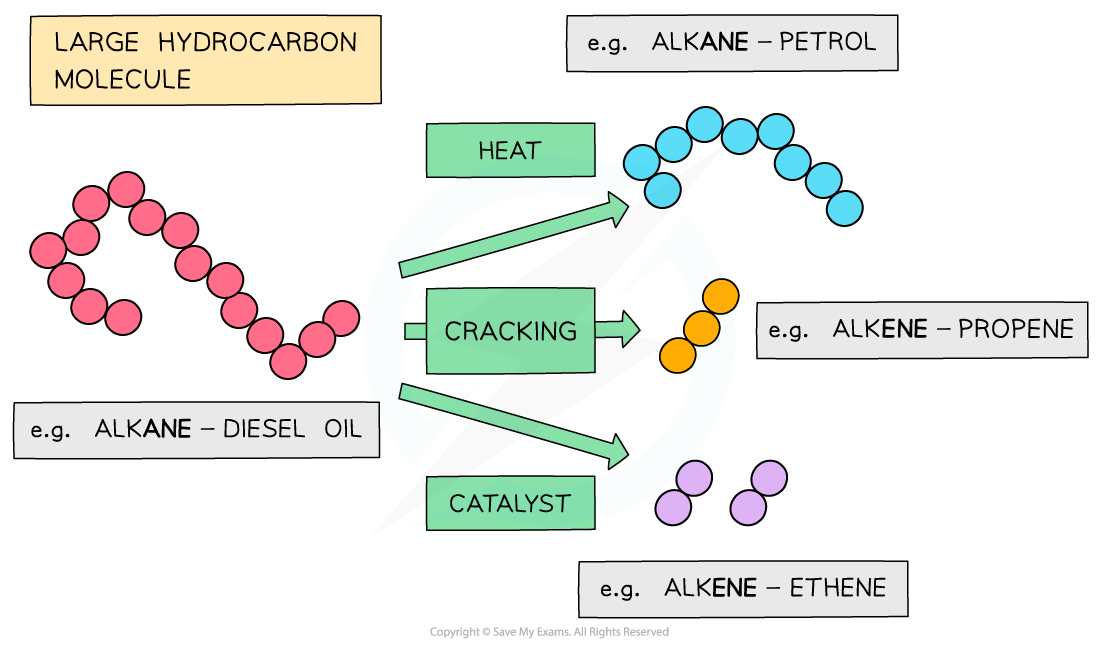
The heavier fractions that are obtained in fractional distillation are further cracked into useful alkane and alkenes with lower Mr values
- When a large hydrocarbon is cracked, a smaller alkane and alkene molecules are formed
- E.g.. octane and ethene from decane
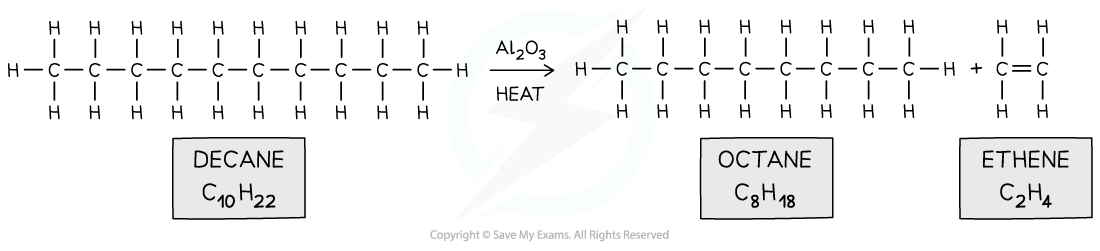
Long hydrocarbon fraction is cracked into two smaller ones
- The low-molecular mass alkanes formed make good fuels and are in high demand
- There are two types of cracking:
- Thermal cracking requires high temperatures (up to 1000 oC) and high pressure (up to 70 atmospheres) and produces alkanes and a lot of alkenes
- Catalytic cracking uses a lower temperature (around 450 oC) and slight pressure in the presence of a catalyst such as a zeolite or aluminium oxide to produce mainly aromatic hydrocarbons
Reforming alkanes
- Many vehicles run on petrol or diesel which are both a mixture of alkanes along with other hydrocarbons, impurities and additives
- Many of the alkanes in these fuels are straight chain alkanes
- These straight chain alkanes are more likely to explode, rather than combust, inside the engine
- This is known as knocking and makes the combustion less efficient
- To reduce this straight chain alkanes are reformed into:
- Branched alkanes, e.g. octane → 2,5-dimethylhexane
CH3CH2CH2CH2CH2CH2CH2CH3 → CH3CH(CH3)CH2CH2CH(CH3)CH3
-
- Cycloalkanes, e.g. hexane → cyclohexane
CH3CH2CH2CH2CH2CH3 → C6H12 + H2
- Reforming often uses a platinum catalyst
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





