- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记2.3.3 Halogen Redox Reactions
Halogen Redox Reactions
Reactions with Group 1 & 2 metals
- The halogens react with some metals to form ionic compounds which are metal halide salts
- In all reactions where halogens are reacting with metals, the metals are being oxidised
- Reaction of sodium and chlorine
- 2Na (s) + Cl2 (g) → 2NaCl (s)
- Na is being oxidised, the oxidation number is changing from 0 to +1
- Calcium is a group 2 metal:
- Ca (s) + Br2 (l) → CaBr2 (s)
- Ca is being oxidised, the oxidation number is changing from 0 to +2
- Therefore the halogens are acting as oxidising agents
Reactions with Iron(II)
- Chlorine and bromine can oxidise iron(II) to iron(III)
Cl2 (g) + 2Fe2+ (aq) → 2Cl- (aq) + 2Fe3+ (aq)
Br2 (g) + 2Fe2+ (aq) → 2Br- (aq) + 2Fe3+ (aq)
- However, iodine is not a strong enough reducing argent to oxidise iron(II) to iron(III)
- Iodine is actually oxidised from iodide ions to iodine by iron(III)
2I- (aq) + 2Fe3+ (aq) → I2 (aq) + 2Fe2+ (aq)
Disproportionation reaction
- A disproportionation reaction is a reaction in which the same species is both oxidised and reduced
- The reaction of chlorine with dilute alkali is an example of a disproportionation reaction
- In these reactions, the chlorine gets oxidised and reduced at the same time
- Different reactions take place at different temperatures of the dilute alkali
Chlorine in cold alkali (15 oC)
- The reaction that takes place is:

- The ionic equation is:
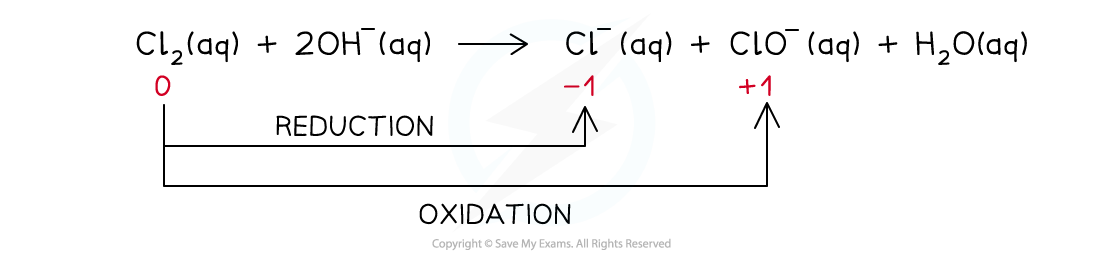
- The ionic equation shows that the chlorine gets both oxidised and reduced
- Chlorine gets oxidised as there is an increase in ox. no. from 0 to +1 in ClO-(aq)
- The half-equation for the oxidation reaction is:

- Chlorine gets reduced as there is a decrease in ox. no. from 0 to -1 in Cl-(aq)
- The half-equation for the reduction reaction is:

Chlorine in hot alkali (70 oC)
- The reaction that takes place is:

- The ionic equation is:
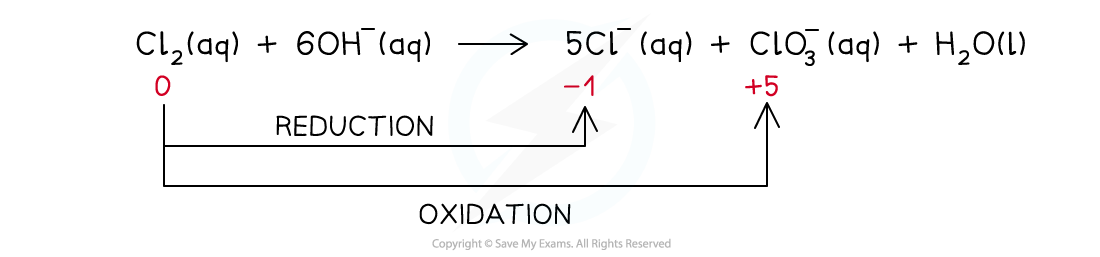
- The ionic equation shows that the chlorine gets both oxidised and reduced
- Chlorine gets oxidised as there is an increase in ox. no. from 0 to +5 in ClO3-(aq)
- The half-equation for the oxidation reaction is:

- Chlorine gets reduced as there is a decrease in ox. no. from 0 to -1 in Cl-(aq)
- The half-equation for the reduction reaction is:

Drinking water
- Chlorine can be used to clean water and make it drinkable
- The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised and reduced

The disproportionation reaction of chlorine with water in which chlorine gets reduced to HCl and oxidised to HClO
- Chloric(I) acid (HClO) sterilises water by killing bacteria
- Chloric acid can further dissociate in water to form ClO-(aq):
HClO (aq) → H+ (aq) + ClO- (aq)
- ClO-(aq) also acts as a sterilising agent cleaning the water
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





