- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记2.1.1 Oxidation Number
Defining Oxidation Number
- There are three definitions of oxidation and reduction used in different branches of chemistry
- Oxidation and reduction can be used to describe any of the following processes
Definitions and Examples of Oxidation & Reduction

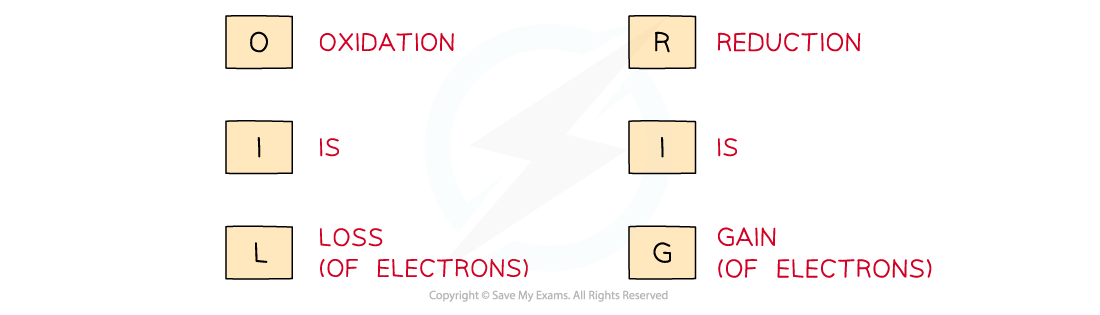
Use the acronym "Oil Rig" to help you remember the definitions of oxidation and reduction
Oxidation Number
- The oxidation number of an atom is the charge that would exist on an individual atom if the bonding were completely ionic
- It is like the electronic ‘status’ of an element
- Oxidation numbers are used to
- Tell if oxidation or reduction has taken place
- Work out what has been oxidised and/or reduced
- Construct half equations and balance redox equations
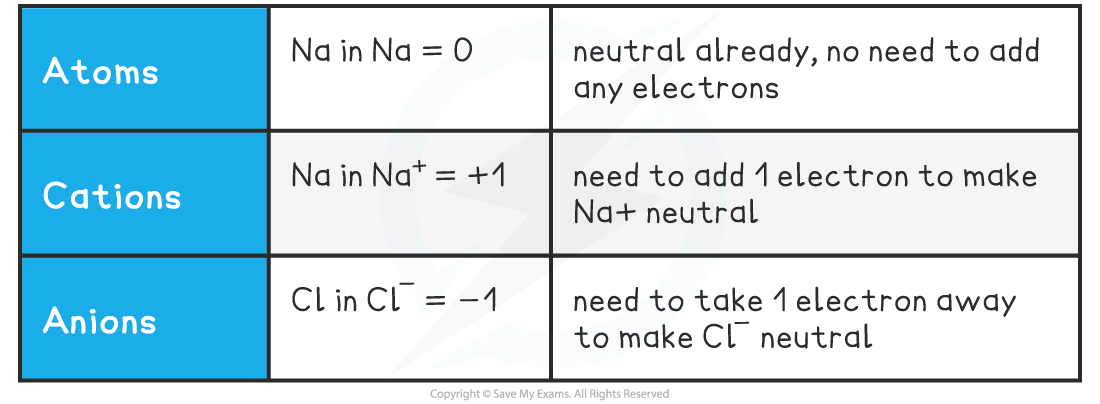
Oxidation Numbers of Simple Ions
Worked Example
What are the oxidation numbers of the elements in the following species?
a) C b) Fe3+ c) Fe2+
d) O2- e) He f) Al3+
Answers:
a) 0 b) +3 c) +2
d) -2 e) 0 f) +3
- So, in simple ions, the oxidation numbers of the atom is the charge on the ion:
- Na+, K+, H+ all have an oxidation number of +1
- Mg2+, Ca2+, Pb2+ all have an oxidation number of +2
- Cl–, Br–, I– all have an oxidation number of -1
- O2-, S2- all have an oxidation number of -2
Calculating Oxidation Numbers
Oxidation Number Rules
- A few simple rules help guide you through the process of determining the oxidation number of any element
- Remember, you are determining the oxidation number of a single atom
- The oxidation number (ox.no.) refers to a single atom in a compound
Oxidation Number Rules Table

Molecules or Compounds
- In molecules or compounds, the sum of the oxidation numbers on the atoms is zero
Oxidation Number in Molecules or Compounds
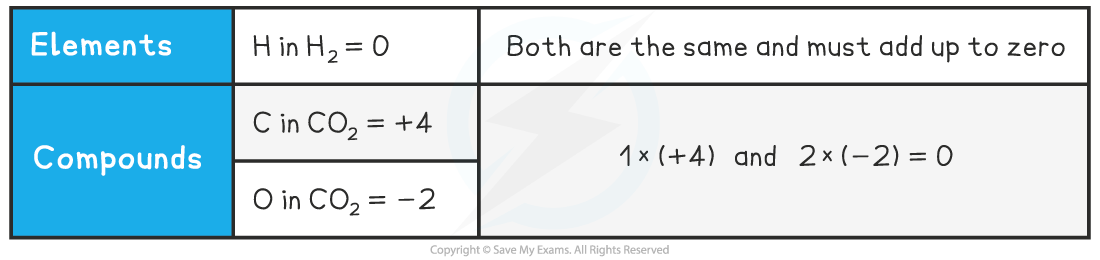
- Because CO2 is a neutral molecule, the sum of the oxidation numbers must be zero
- For this, one element must have a positive oxidation number and the other must be negative
How do you determine which is the positive one?
- The more electronegative species will have the negative value
- Electronegativity increases across a period and decreases down a group
- O is further to the right than C in the periodic table so it has the negative value
How do you determine the value of an element’s oxidation number?
- From its position in the periodic table and / or
- The other element(s) present in the formula
- The oxidation numbers of all other atoms in their compounds can vary
- By following the oxidation number rules, the oxidation number of any atom in a compound or ion can be deduced
- The position of an element in the periodic table can act as a guide to the oxidation number
Oxidation Numbers & the Periodic Table

转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





