- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.8.3 Calorimetry
Calculating Energy Transferred, Q
Measuring enthalpy changes
- Calorimetry is the measurement enthalpy changes in chemical reactions
- A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can
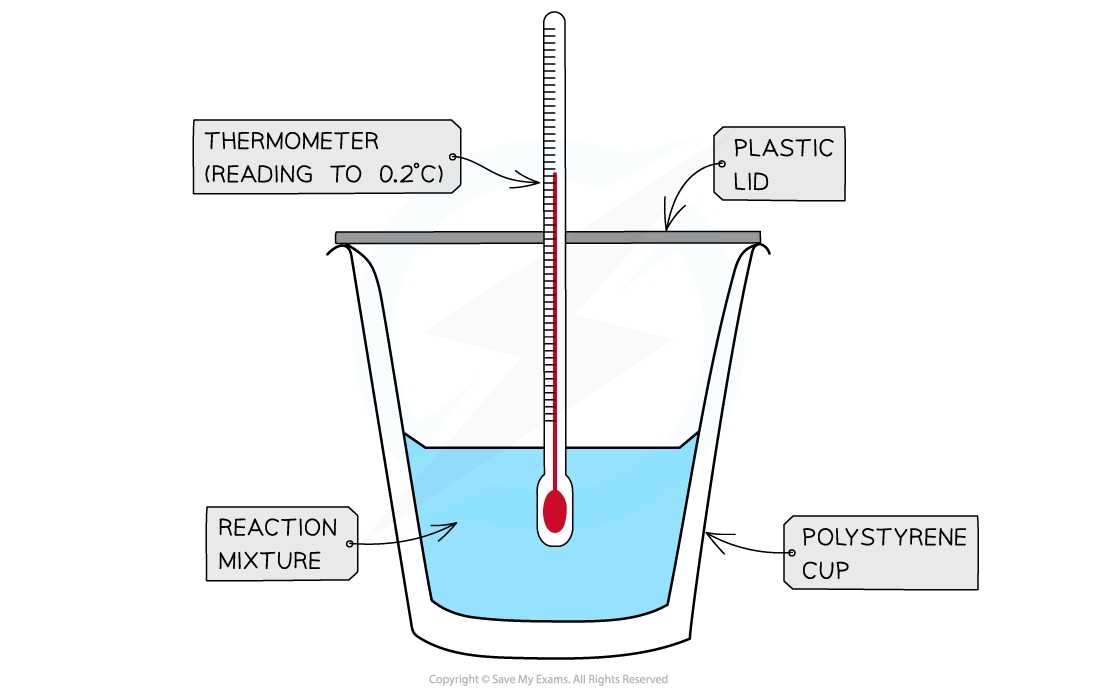
A polystyrene cup can act as a calorimeter to find enthalpy changes in a chemical reaction
- The energy needed to increase the temperature of 1 g of a substance by 1 oC is called the specific heat capacity (c ) of the liquid
- The specific heat capacity of water is 4.18 J g-1 K-1
- The energy transferred as heat can be calculated by:
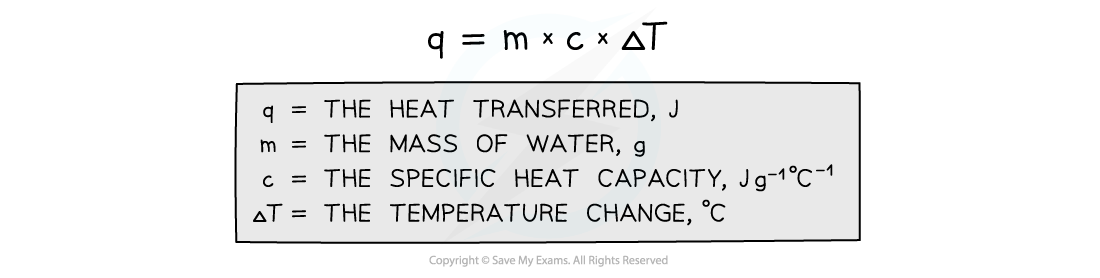
Equation for calculating energy transferred in a calorimeter
Worked Example
Specific heat capacity calculations
In a calorimetry experiment 2.50 g of methane is burnt in excess oxygen.
30% of the energy released during the combustion is absorbed by 500 g of water, the temperature of which rises from 25 °C to 68 °C.
The specific heat capacity of water is 4.18 J g-1 K−1
What is the total energy released per gram of methane burnt?
Answer
Step 1
-
- q = m x c x ΔT
- m (of water) = 500 g
- c (of water) = 4.18 J g-1 °C-1
- ΔT (of water) = 68 oC - 25 oC = 43 oC
Step 2:
-
- q = 500 x 4.18 x 43 = 89 870 J
Step 3:
-
- This is only 30% of the total energy released by methane
- Total energy x 0.3 = 89 870 J
- Total energy = 299 567 J
Step 4:
-
- This is released by 2.50 g of methane
- Energy released by 1.00 g of methane = 299 567 ÷ 2.50 = 120 000 J g-1 (to 3 s.f.) or 120 kJ g-1
Calculating Enthalpy Changes
- Aqueous solutions of acid, alkalis and salts are assumed to be largely water so you can just use the m and c values of water when calculating the energy transferred.
- To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used:
ΔH = 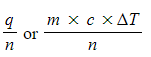
- When there is a rise in temperature, the value for ΔH becomes negative suggesting that the reaction is exothermic
- This means that your value should be negative for an exothermic reaction, e.g. combustion
- When the temperature falls, the value for ΔH becomes positive suggesting that the reaction is endothermic
- This means that your value should be positive for an endothermic reaction, e.g. combustion
Worked Example
1.50 g of an organic liquid (Mr = 58.0) underwent complete combustion. The heat formed raised the temperature of 100 g of water from 20 oC to 75 oC.
Calculate the enthalpy of combustion for the organic liquid
Answer
Step 1: Calculate the energy released by the organic liquid
-
- Q = mcΔT
- Q = 100 x 4.18 x (75 - 20)
- Q = 22990 J
- Q = 22.99 kJ
Step 2: Calculate the number of moles of the organic liquid
-
- Number of moles =
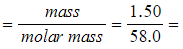 0.0259 moles (to 3s.f.)
0.0259 moles (to 3s.f.)
- Number of moles =
Step 3: Calculate the enthalpy change of combustion
-
- ΔcHθ
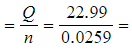 -887 kJ mol-1 (to 3s.f.)
-887 kJ mol-1 (to 3s.f.)
- Remember, combustion is an exothermic process and will, therefore, be a negative enthalpy change value
- ΔcHθ
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





