- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.7.2 Titrations
Titration Calculations
Volumetric Analysis
- Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of another unknown solution
- The technique most commonly used is a titration
- The volumes are measured using two precise pieces of equipment, a volumetric or graduated pipette and a burette
- Before the titration can be done, the standard solution must be prepared
- Specific apparatus must be used both when preparing the standard solution and when completing the titration, to ensure that volumes are measured precisely
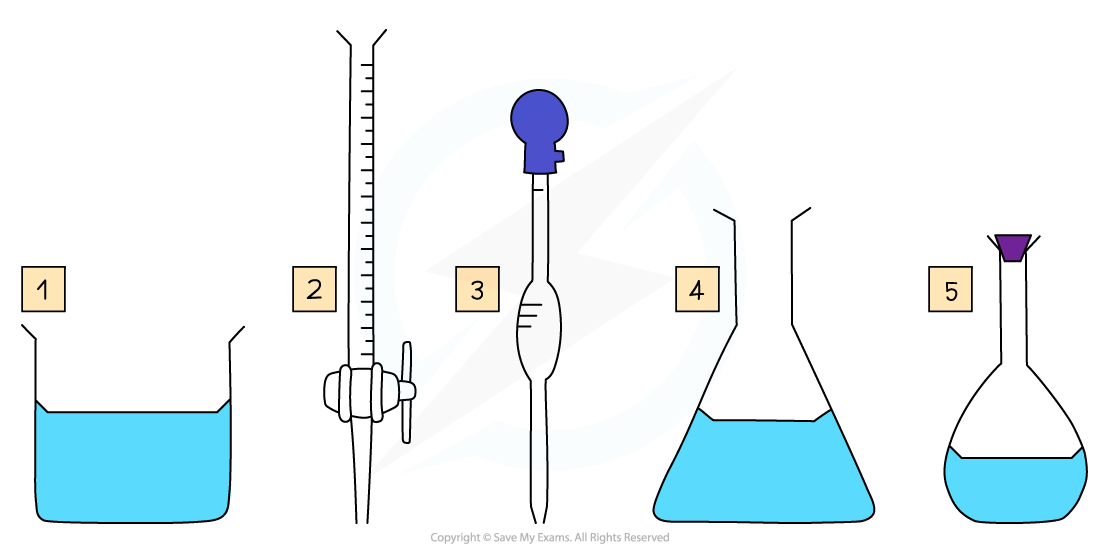
Some key pieces of apparatus used to prepare a volumetric solution and perform a simple titration
- Beaker
- Burette
- Volumetric Pipette
- Conical Flask
- Volumetric Flask
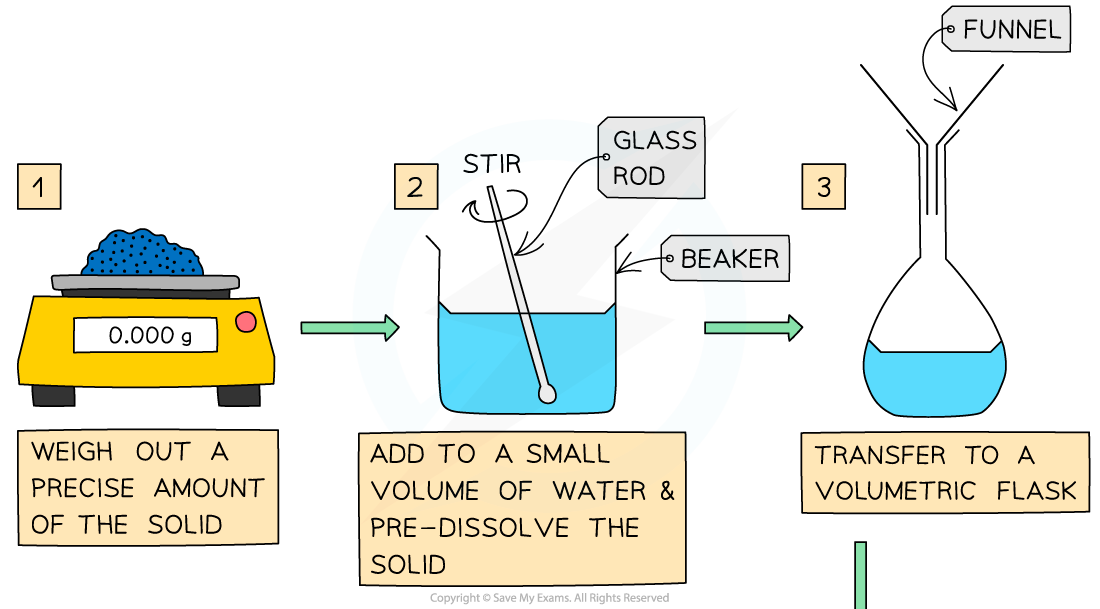
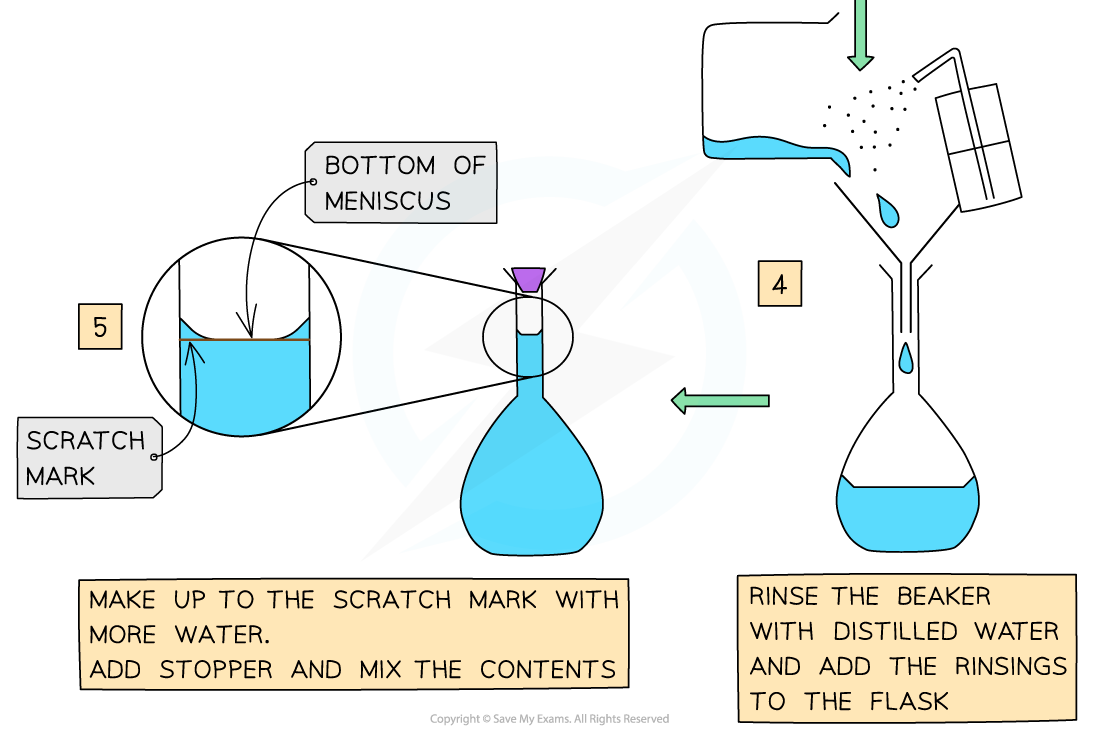
Making a Standard Solution
- Chemists routinely prepare solutions needed for analysis, whose concentrations are known precisely
- These solutions are termed volumetric solutions or standard solutions
- They are made as accurately and precisely as possible using three decimal place balances and volumetric flasks to reduce the impact of measurement uncertainties
- The steps are:
Performing the Titration
- The key piece of equipment used in the titration is the burette
- Burettes are usually marked to a precision of 0.10 cm3
- Since they are analogue instruments, the uncertainty is recorded to half the smallest marking, in other words to ±0.05 cm3
- The end point or equivalence point occurs when the two solutions have reacted completely and is shown with the use of an indicator
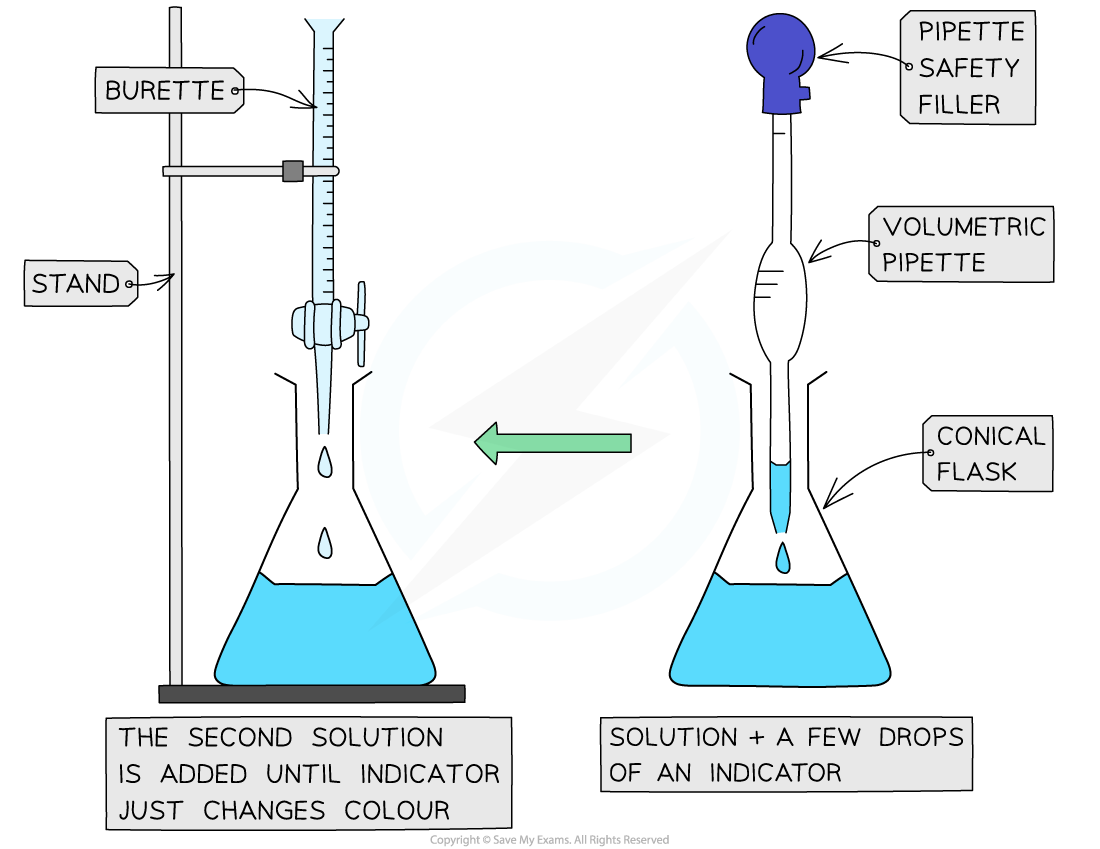
The steps in a titration
- A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour change
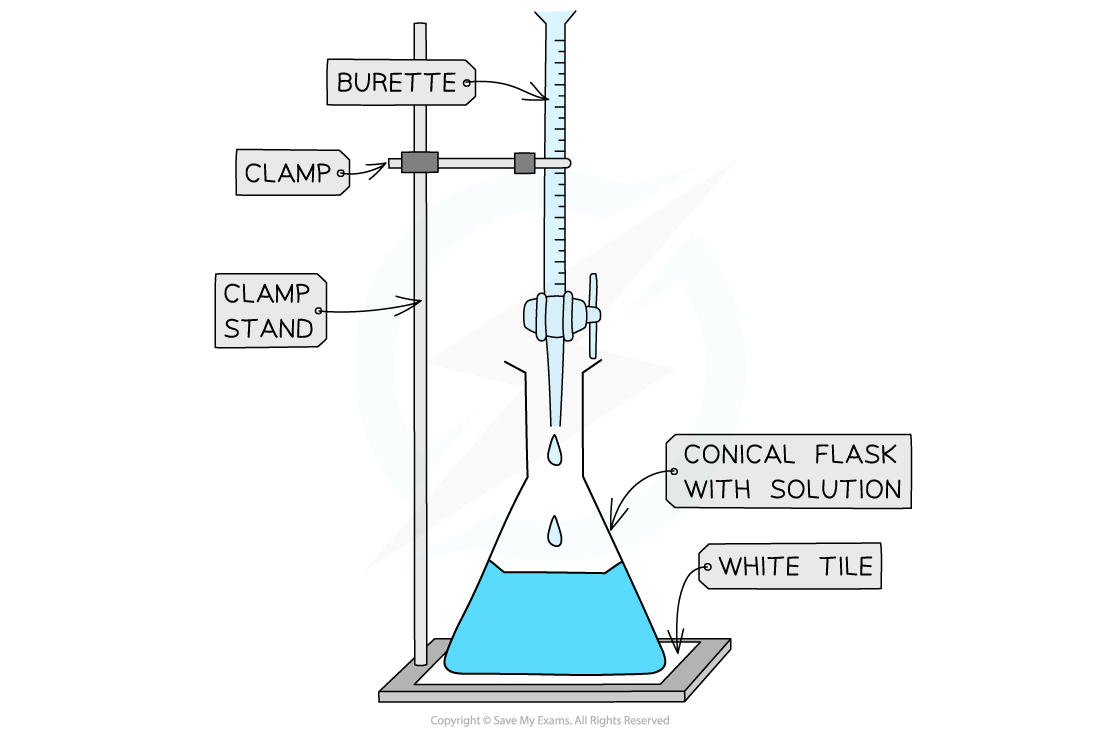
The steps in a titration
- The steps in a titration are:
- Measuring a known volume (usually 20 or 25 cm3) of one of the solutions with a volumetric pipette and placing it into a conical flask
- The other solution is placed in the burette
- To start with, the burette will usually be filled to 0.00 cm3
- A few drops of the indicator are added to the solution in the conical flask
- The tap on the burette is carefully opened and the solution added, portion by portion, to the conical flask until the indicator starts to change colour
- As you start getting near to the end point, the flow of the burette should be slowed right down so that the solution is added dropwise
- You should be able to close the tap on the burette after one drop has caused the colour change
- Multiple runs are carried out until concordant results are obtained
- Concordant results are within 0.1 cm3 of each other
Recording and processing titration results
- Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm3, the same as the uncertainty
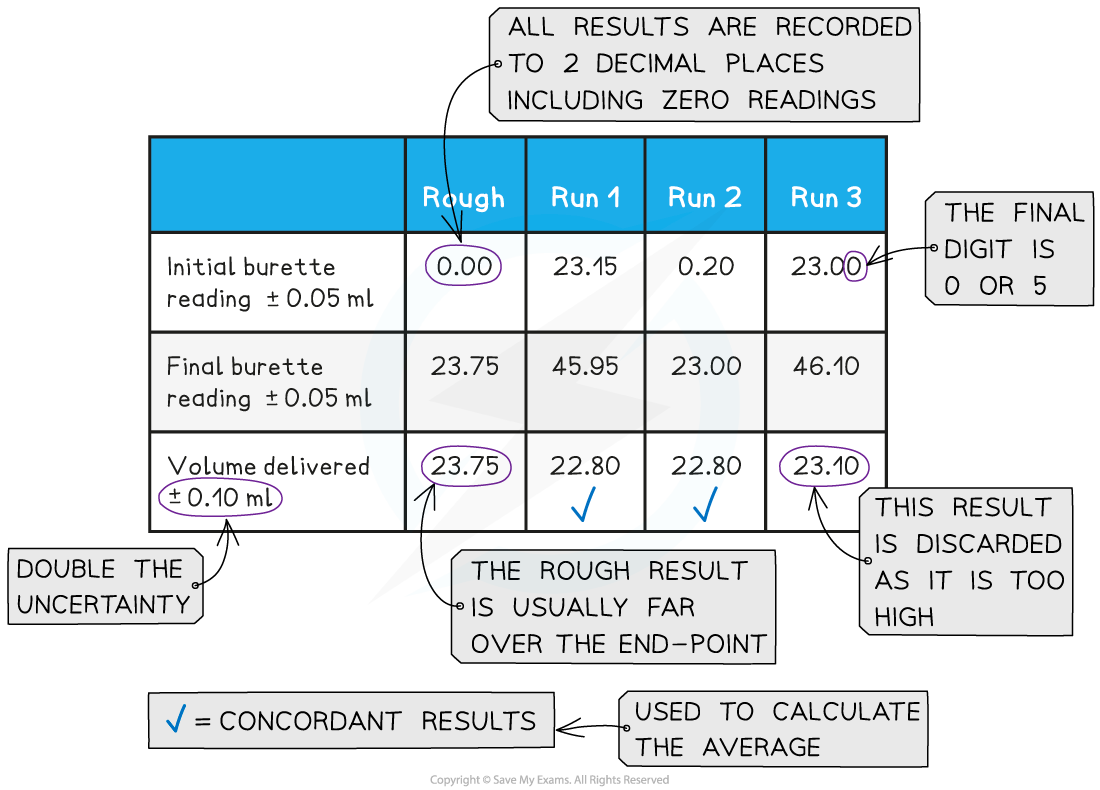
A typical layout and set of titration results
- The volume delivered (titre) is calculated and recorded to an uncertainty of ±0.10 cm3
- The uncertainty is doubled, because two burette readings are made to obtain the titre (V final – V initial), following the rules for propagation of uncertainties
- Concordant results are then averaged, and non-concordant results are discarded
- The appropriate calculations are then done
Volumes & concentrations of solutions
- The concentration of a solution is the amount of solute dissolved in a solvent to make 1 dm3 of solution
- The solute is the substance that dissolves in a solvent to form a solution
- The solvent is often water
Concentration (mol dm-3) = 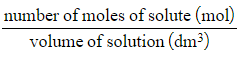
- A concentrated solution is a solution that has a high concentration of solute
- A dilute solution is a solution with a low concentration of solute
- When carrying out calculations involve concentrations in mol dm-3 the following points need to be considered:
- Change mass in grams to moles
- Change cm3 to dm3
- To calculate the mass of a substance present in solution of known concentration and volume:
- Rearrange the concentration equation
number of moles (mol) = concentration (mol dm-3) x volume (dm3)
-
- Multiply the moles of solute by its molar mass
mass of solute (g) = number of moles (mol) x molar mass (g mol-1)
Worked Example
Neutralisation calculation
25.0 cm3 of 0.050 mol dm-3 sodium carbonate was completely neutralised by 20.00 cm3 of dilute hydrochloric acid. Calculate the concentration in mol dm-3 of hydrochloric acid.
Answer
Step 1: Write the balanced symbol equation
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
Step 2: Calculate the amount, in moles, of sodium carbonate reacted by rearranging the equation for amount of substance (mol) and dividing the volume by 1000 to convert cm3 to dm3
-
- Amount (Na2CO3) = 0.025 dm3 x 0.050 mol dm-3 = 0.00125 mol
Step 3: Calculate the moles of hydrochloric acid required using the reaction’s stoichiometry
-
- 1 mol of Na2CO3 reacts with 2 mol of HCl, so the molar ratio is 1 : 2
- Therefore 0.00125 moles of Na2CO3 react with 0.00250 moles of HCl
Step 4: Calculate the concentration, in mol dm-3, of hydrochloric acid
-
- [HCl]
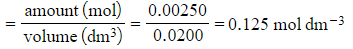
- [HCl]
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





