- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.6.4 The Mole & the Avogadro Constant
The Mole & Avogadro
- The Avogadro constant (NA or L) is the number of particles equivalent to the relative atomic mass or molecular mass of a substance
- The Avogadro constant applies to atoms, molecules, ions and electrons
- The value of NA is 6.02 x 1023 g mol-1
- The mass of a substance with this number of particles is called a mole (mol)
- This can be called the molar mass
- This is the mass of substance that contains the same number of fundamental units as exactly 12.00g of carbon-12
- The amount / number of moles of a substance, n, the mass of the substance, m, and the molar mass, M, are linked by the equation:
n = ![]()
- One mole of any element is equal to the relative atomic mass of that element in grams
- If you had one mole of carbon in your hand, that is 6.02 x 1023 atoms of carbon, you would have a mass of 12.00 g
- One mole of water would have a mass of (2 x 1 + 16) = 18 g
Worked Example
- What is the molar mass of water?
- How many moles are there in 100 g of water?
- How many water molecules are there in 100 g of water?
Answers
-
- Molar mass of water, H2O = (2 x 1.0) + 16.0 = 18.0 g mol-1
- Moles
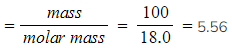 moles (to 3 s.f.)
moles (to 3 s.f.) - Number of molecules = number of moles x Avogadro's constant = 5.56 x (6.02 x 1023) = 3.35 x 1024 molecules
Worked Example
What is the mass of the following:
- Five hundred million atoms of platinum
- (1.31 x 1022) molecules of ethanol
Answer 1:
Number of moles 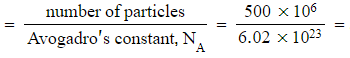 8.31 x10-16 moles
8.31 x10-16 moles
Mass = moles x molar mass = (8.31 x10-16) x 195.1 = 1.62 x 10-13 g (lots of atoms, a tiny mass)
Answer 2:
Molar mass of ethanol, C2H5OH = (2 x 12.0) + (5 x 1.0) + 16.0 + 1.0 = 46.0 g mol-1
Number of moles 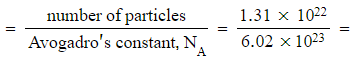 0.0218 moles
0.0218 moles
Mass = moles x molar mass = 0.0218 x 46.0 = 1.00 g (an exceptionally large number of molecules in just 1 g)
Exam Tip
- When you are completing calculations using Avogadro's constant, you may end up with answers that seem very large or very small - don't automatically assume that they must be wrong
- Remember, Avogadro's constant is a VERY large number:
- 6.02 x 1023 or 602 000 000 000 000 000 000 000
- So when you multiply or divide by Avogadro's constant, your answers will, naturally, become very large or very small
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





