- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.6.2 Balanced Equations
Writing Balanced Equations
- A symbol equation is a shorthand way of describing a chemical reaction using chemical symbols to show the number and type of each atom in the reactants and products
- A word equation is a longer way of describing a chemical reaction using only words to show the reactants and products
Balancing equations
- During chemical reactions, atoms cannot be created or destroyed
- The number of each atom on each side of the reaction must therefore be the same
- E.g. the reaction needs to be balanced
- When balancing equations remember:
- Not to change any of the formulae
- To put the numbers used to balance the equation in front of the formulae
- To balance firstly the carbon, then the hydrogen and finally the oxygen in combustion reactions of organic compounds
- When balancing equations follow the following the steps:
- Write the formulae of the reactants and products
- Count the numbers of atoms in each reactant and product
- Balance the atoms one at a time until all the atoms are balanced
- Use appropriate state symbols in the equation
- The physical state of reactants and products in a chemical reaction is specified by using state symbols
- (s) solid
- (l) liquid
- (g) gas
- (aq) aqueous
Worked Example
Balance the following equation:
magnesium + oxygen → magnesium oxide
Answer:
Step 1: Write out the symbol equation showing reactants and products
Mg + O2 → MgO
Step 2: Count the numbers of atoms in each reactant and product
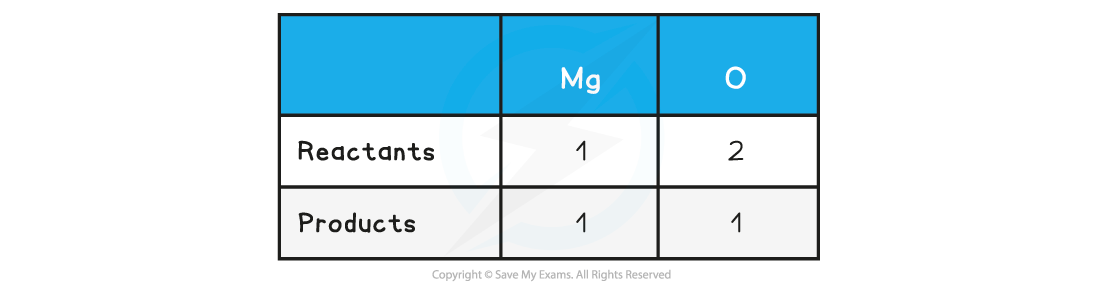
Step 3: Balance the atoms one at a time until all the atoms are balanced
2Mg + O2 → 2MgO
This is now showing that 2 moles of magnesium react with 1 mole of oxygen to form 2 moles of magnesium oxide
Step 4: Use appropriate state symbols in the fully balanced equation
2Mg (s) + O2 (g) → 2MgO (s)
Ionic equations
- In aqueous solutions ionic compounds dissociate into their ions
- Many chemical reactions in aqueous solutions involve ionic compounds, however only some of the ions in solution take part in the reactions
- The ions that do not take part in the reaction are called spectator ions
- An ionic equation shows only the ions or other particles taking part in a reaction, and not the spectator ions
Worked Example
1. Balance the following equation
zinc + copper(II) sulfate → zinc sulfate + copper
2. Write down the ionic equation for the above reaction
Answer 1:
Step 1: To balance the equation, write out the symbol equation showing reactants and products
Zn + CuSO4 → ZnSO4 + Cu
Step 2: Count the numbers of atoms in each reactant and product. The equation is already balanced
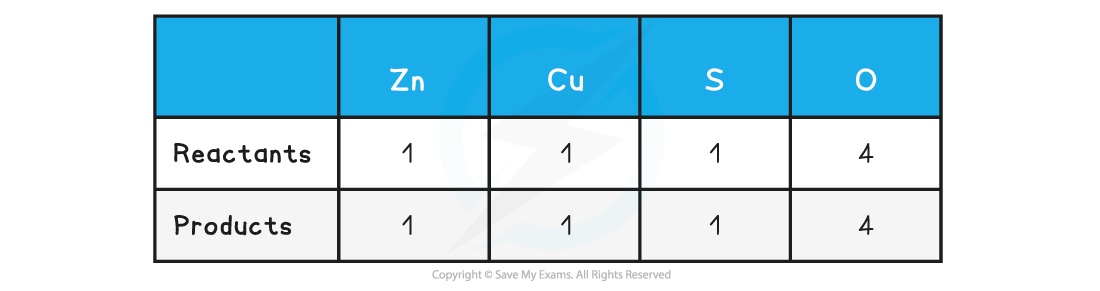
Step 3: Use appropriate state symbols in the equation
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
Answer 2:
Step 1: The full chemical equation for the reaction is
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
Step 2: Break down reactants into their respective ions
Zn (s) + Cu2+ + SO42- (aq) → Zn2++ SO42- (aq) + Cu (s)
Step 3: Cancel the spectator ions on both sides to give the ionic equation
Zn (s) + Cu2+ + SO42- (aq) → Zn2++ SO42- (aq) + Cu (s)
Zn (s) + Cu2+(aq) → Zn2+ (aq) + Cu (s)
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





