- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.5.8 Predicting Structures
Predicting Structure & Bonding
- Different types of structure and bonding have different effects on the physical properties of substances such as their melting and boiling points, electrical conductivity and solubility
Characteristics of Different Compound Structure Types Table
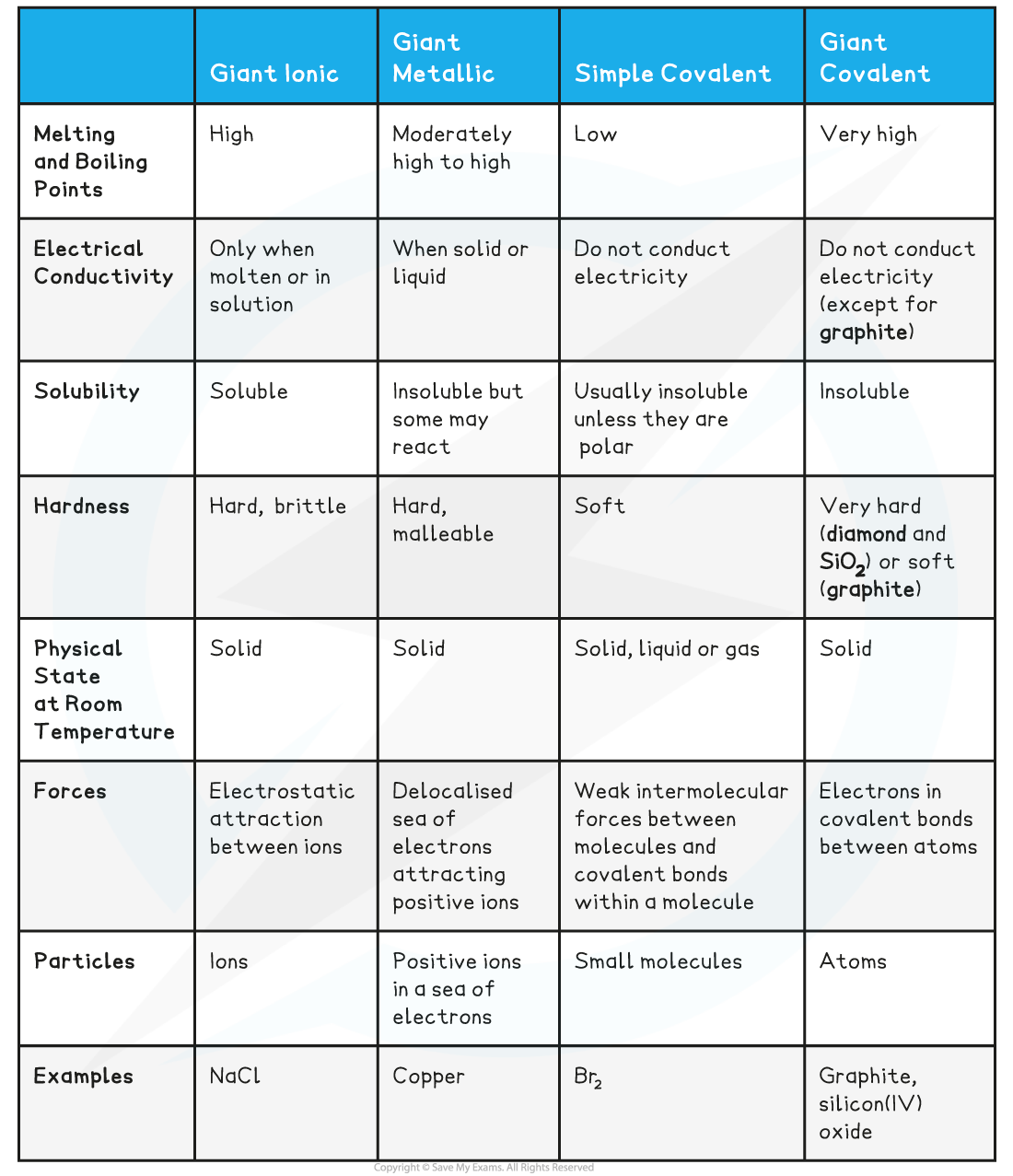
Predicting Physical Properties
Worked Example
Bonding & structure
The table below shows the physical properties of substances X, Y and Z
Which one of the following statements about X and Y is completely true?
Statement 1: X has a giant ionic structure, Y has a giant molecular structure, Z is a metal
Statement 2: X is a metal, Y has a simple molecular structure, Z has a giant molecular structure
Statement 3: X is a metal, Y has a simple molecular structure, Z has a giant ionic structure
Statement 4: X has a giant ionic structure, Y has a simple molecular structure, Z is a metal
Answer
The correct answer is Statement 4
-
- The relatively high melting point, solubility in water and electrical conductivity when molten suggest that X is a giant ionic structure.
- The low melting point of Y suggests that little energy is needed to break the lattice which corresponds to a simple molecular structure. This is further supported by the low electrical conductivity and its being almost insoluble in water.
- Compound Z has a very high melting point which is characteristic of either metallic or giant molecular lattices, however since it conducts electricity, compound Z must be a giant metallic lattice.
Worked Example
Bonding & structure
Compound X has the following properties: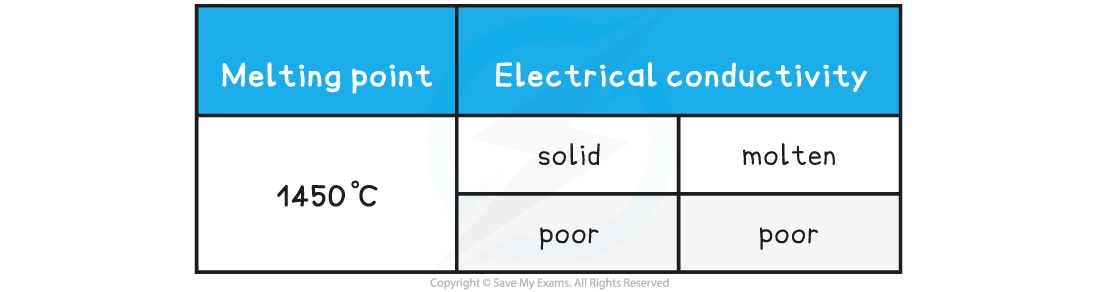
What is the most probable structure of X?
A. Network covalent
B. Polar covalent molecule
C. Ionic lattice
D. Metallic lattice
Answer:
The correct option is A
-
- A high melting point is characteristic of a giant structure, which could be metallic, ionic or covalent
- The poor conductivity as a liquid and solid would match a giant covalent or network covalent structure
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





