- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.5.7 Covalent Structures
Simple Molecular Compounds
Simple Molecular Compounds
- Covalent substances tend to have small molecular structures, such as Cl2, H2O or CO2
- These small molecules are known as simple molecules
- Hydrogen (H2), chlorine (Cl2), oxygen (O2), nitrogen (N2), hydrogen chloride (HCl), water (H2O), ammonia (NH3) and methane (CH4) are also examples of simple molecules
- Iodine is a simple molecule and can be represented but it exists as a crystalline structure involving a regular structure held together by weak London dispersion forces
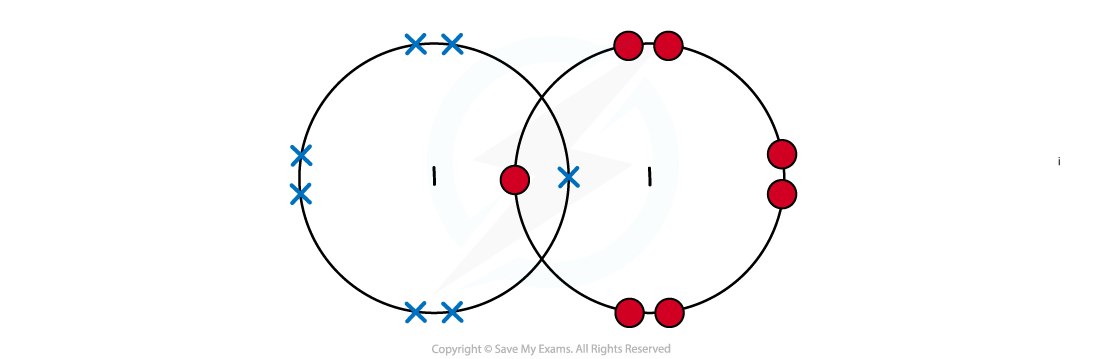
Dot cross diagram of an iodine molecule
Carbon Allotropes
Covalent bonding & giant covalent lattice structures
- Giant covalent lattices have very high melting and boiling points
- These compounds have a large number of covalent bonds linking the whole structure
- A lot of energy is required to break the lattice
- The compounds can be hard or soft
- Graphite is soft as the forces between the carbon layers are weak
- Diamond and silicon(IV) oxide are hard as it is difficult to break their 3D network of strong covalent bonds
- Most compounds are insoluble with water
- Most compounds do not conduct electricity however some do
- Graphite has delocalised electrons between the carbon layers which can move along the layers when a voltage is applied
- Diamond and silicon(IV) oxide do not conduct electricity as all four outer electrons on every carbon atom are involved in a covalent bond so there are no freely moving electrons available
Graphite
- Each carbon atom in graphite is bonded to three others forming layers of hexagons, leaving one free electron per carbon atom
- These free electrons migrate along the layers and are free to move and carry charge, hence graphite can conduct electricity
- The covalent bonds within the layers are very strong, but the layers are attracted to each other by weak intermolecular forces, so the layers can slide over each other making graphite soft and slippery
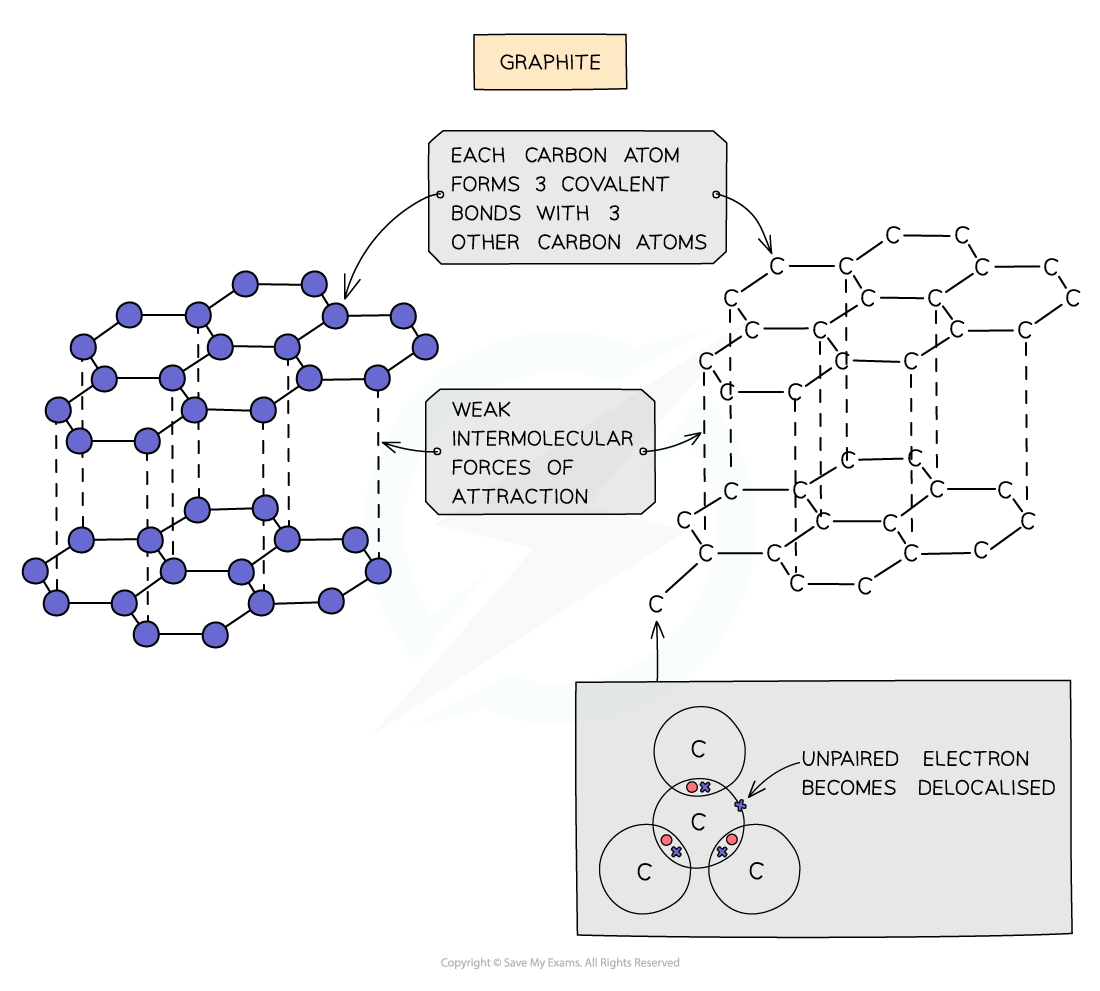
Diagram showing the structure and bonding arrangement in graphite
Diamond
- In diamond, each carbon atom bonds with four other carbons, forming a tetrahedron
- All the covalent bonds are identical, very strong and there are no intermolecular forces
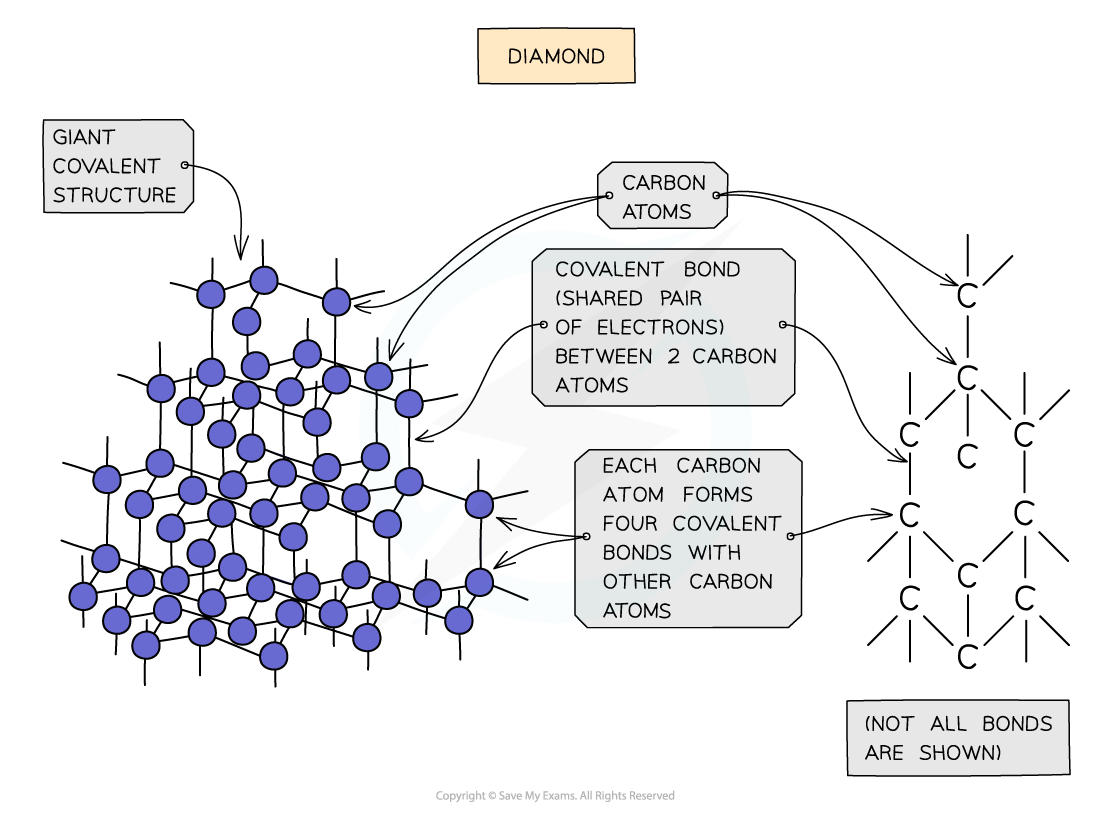
Diagram showing the structure and bonding arrangement in diamond
Graphene
- Graphene consists of a single layer of graphite which is a sheet of carbon atoms covalently bonded forming a continuous hexagonal layer
- It is essentially a 2D molecule since it is only one atom thick
- It has very unusual properties make it useful in fabricating composite materials and in electronics
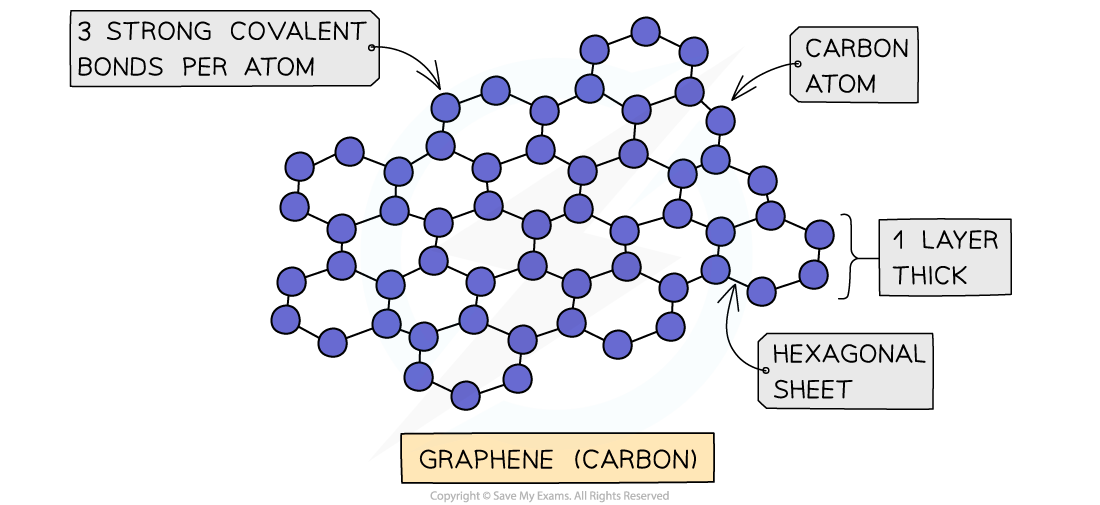
Graphene is a truly remarkable material that has some unexpected properties
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





