- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.5.6 Giant Lattices
Giant Lattices
Ionic Lattices
- The ions form a lattice structure which is an evenly distributed crystalline structure
- Ions in a lattice are arranged in a regular repeating pattern so that positive charges cancel out negative charges
- The attraction between the cations and anions is occurring in all directions
- Each ion is attracted to all of the oppositely charged ions around it
- Therefore the final lattice is overall electrically neutral
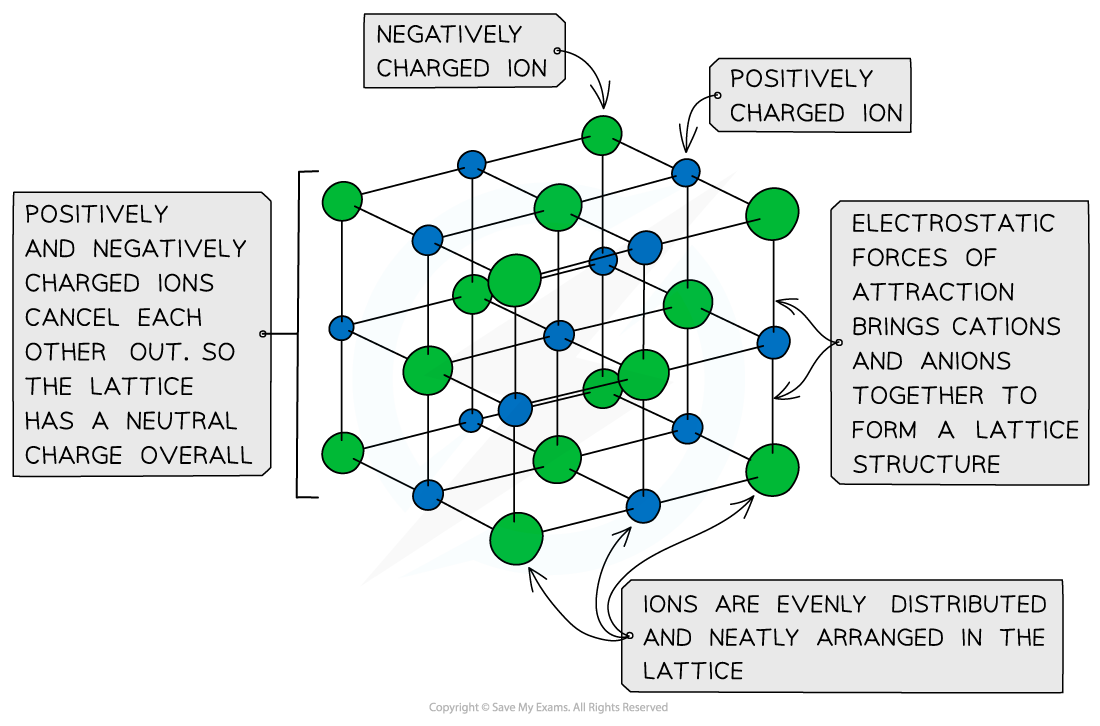
Ionic solids are arranged in lattice structures
Metallic Lattices
- Metals form giant metallic lattices in which the metal ions are surrounded by a ‘sea’ of delocalised electrons
- The metal ions are often packed in hexagonal layers or in a cubic arrangement
- This layered structure with the delocalised electrons gives a metal its key properties
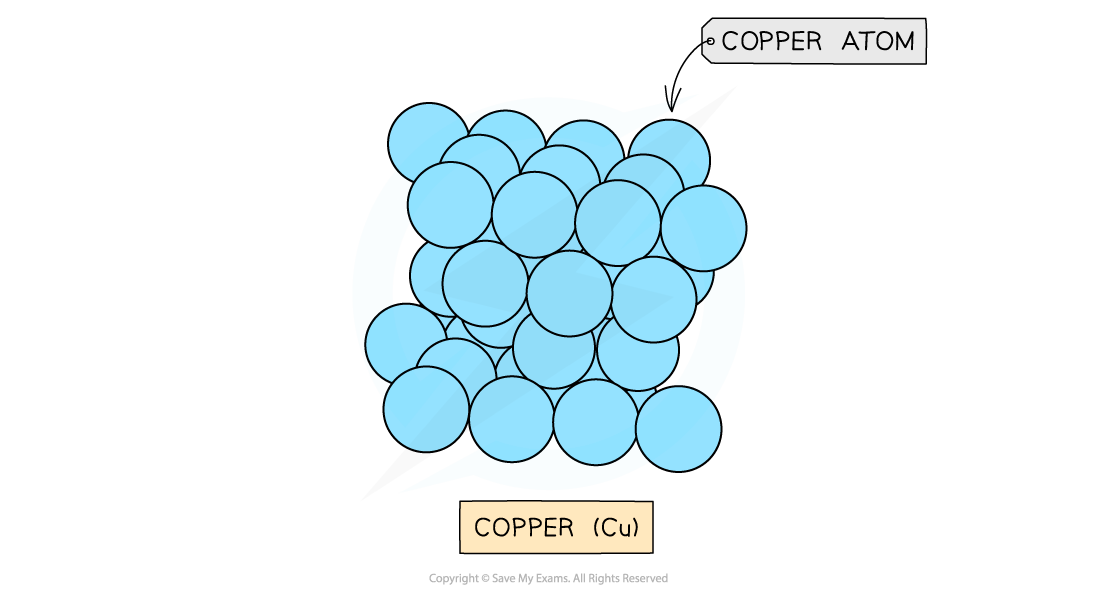
Layers of copper ions (the delocalised electrons are not shown in the diagram)
Covalent lattices
- Covalent bonds are bonds between nonmetals in which electrons are shared between the atoms
- Covalent compounds can be arranged in simple molecular or giant molecular lattices
- Simple molecular lattices: iodine, buckminsterfullerene (C60) and ice
- Giant molecular: silicon(IV) oxide, graphite and diamond
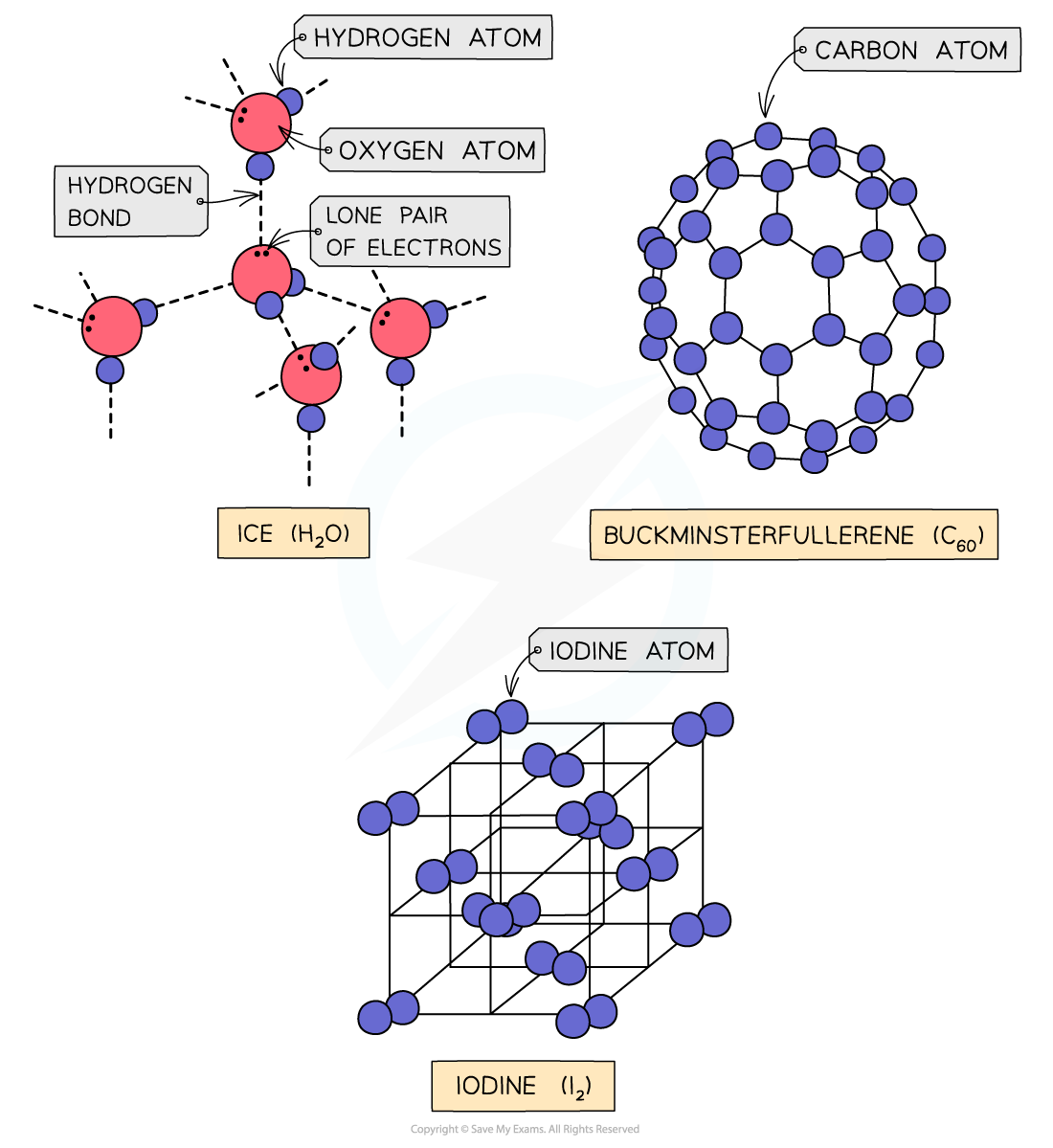
Simple molecular lattices
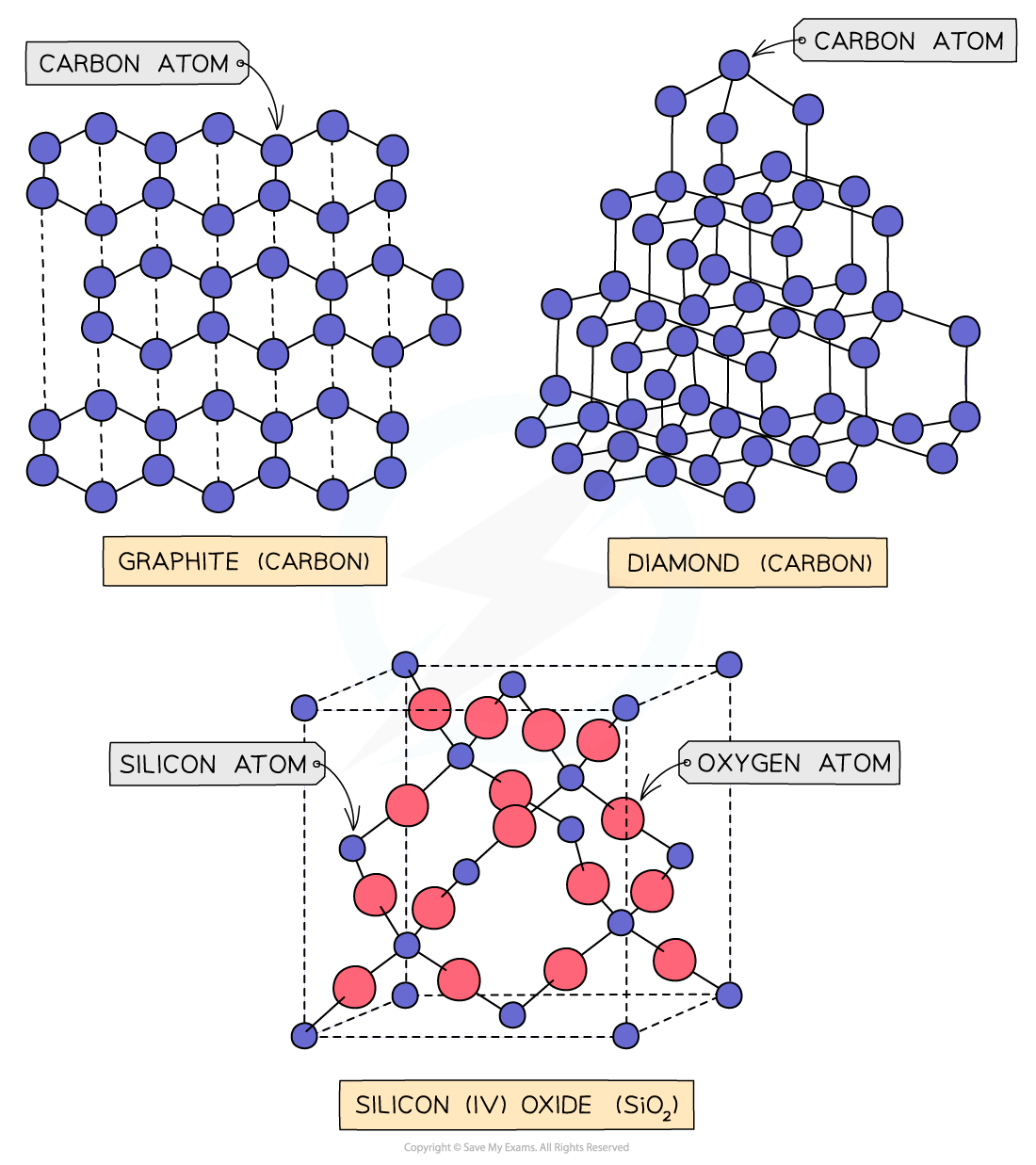
Giant molecular lattices
Exam Tip
Graphite, diamond and buckminsterfullerene are all allotropes of carbon; they are different structural forms of the same element (which is carbon).
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





