- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.4.4 Physical Properties of Ionic Compounds
Physical Properties of Ionic Compounds
- Most ionic, metallic and covalent solids are crystalline lattices
- The ions, atoms or molecules are arranged in a regular and repeating arrangement
Giant ionic lattices
- An ionic bond is an electrostatic force of attraction between a positively charged metal (cation) ion and a negatively charged non-metal (anion) ion
- The metal becomes positively charged as it transfers electrons to the non-metal which then becomes negatively charged
- When an ionic compound is formed, the attraction between the ions happens in all directions
- Ionic compounds are arranged in giant ionic lattices (also called giant ionic structures)
- The type of lattice formed depends on the sizes of the positive and negative ions which are arranged in an alternating fashion
- The ionic lattice of MgO and NaCl are cubic
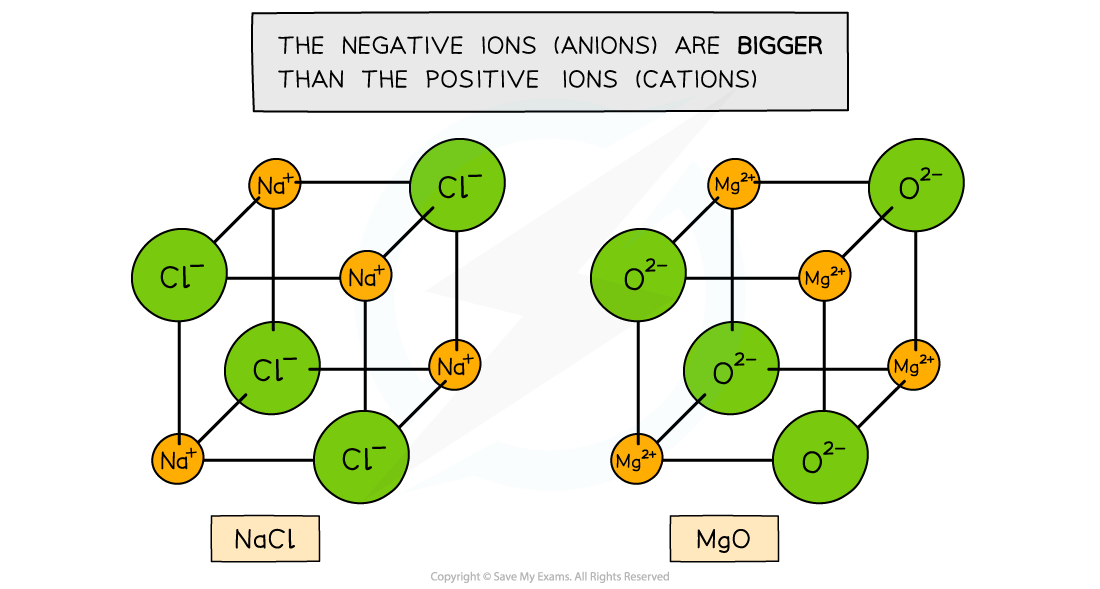
Ionic lattices of the ionic compounds NaCl and MgO
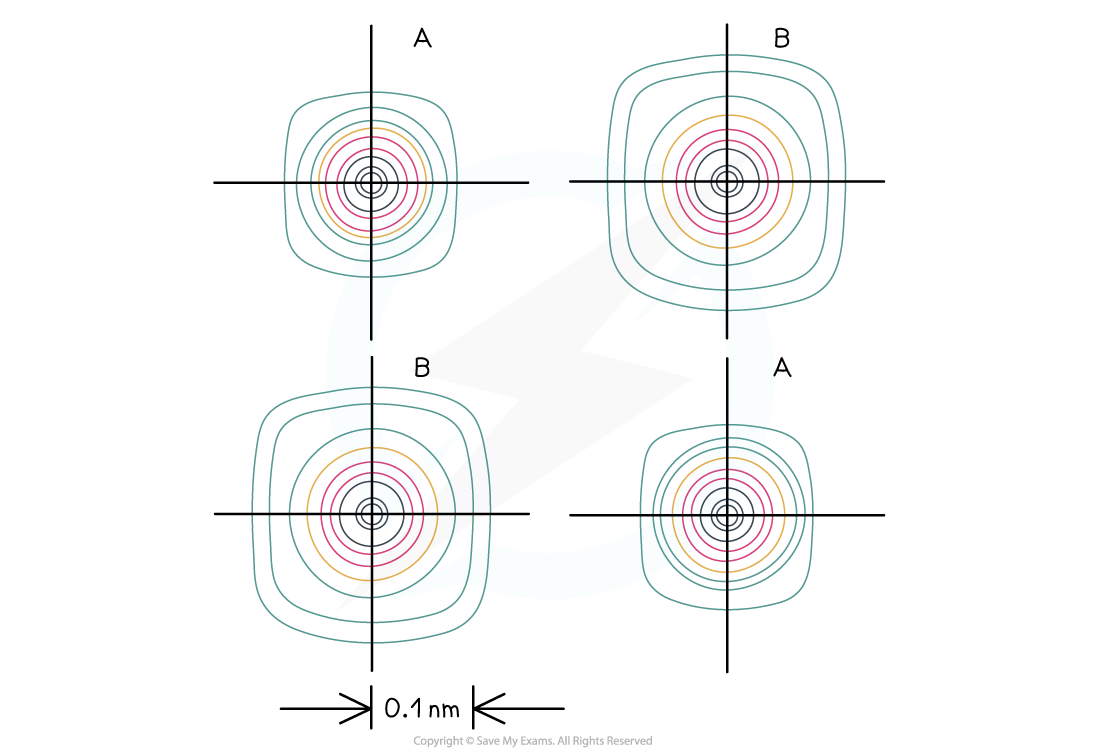
Electron density maps shown above show the likelihood of finding electrons in a region. This shows the electron density map for NaCl. The contours are lines of equal electron density. A = Na+ ions (smaller), B = Cl- ions (larger). Between the ions the electron density falls to zero.
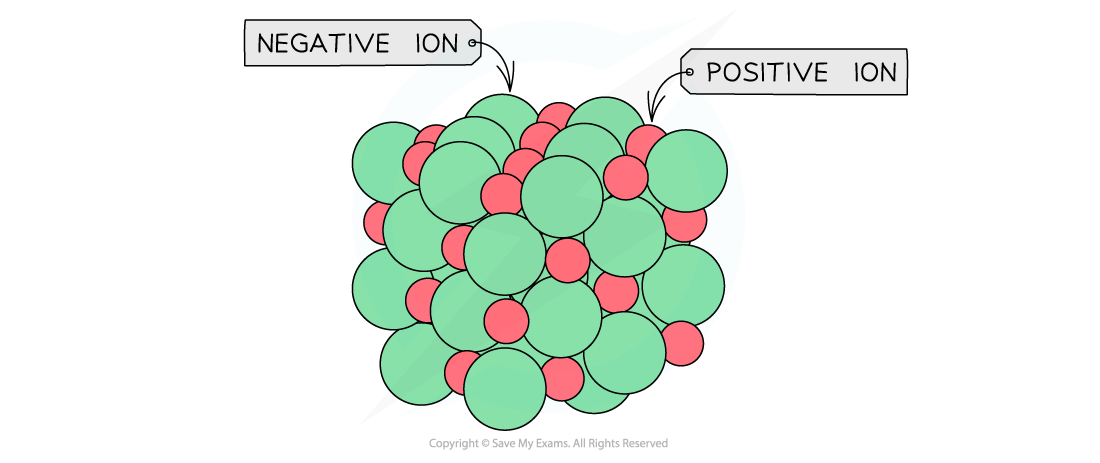
General ionic lattice which shows the actual packing of the ions
Evidence
- The behaviour of ionic substances during electrolysis is a clear piece of evidence for the existence of ions
- Positive ions in solution are attracted to the negative electrode
- Negative ions in solution are attracted to the positive electrode
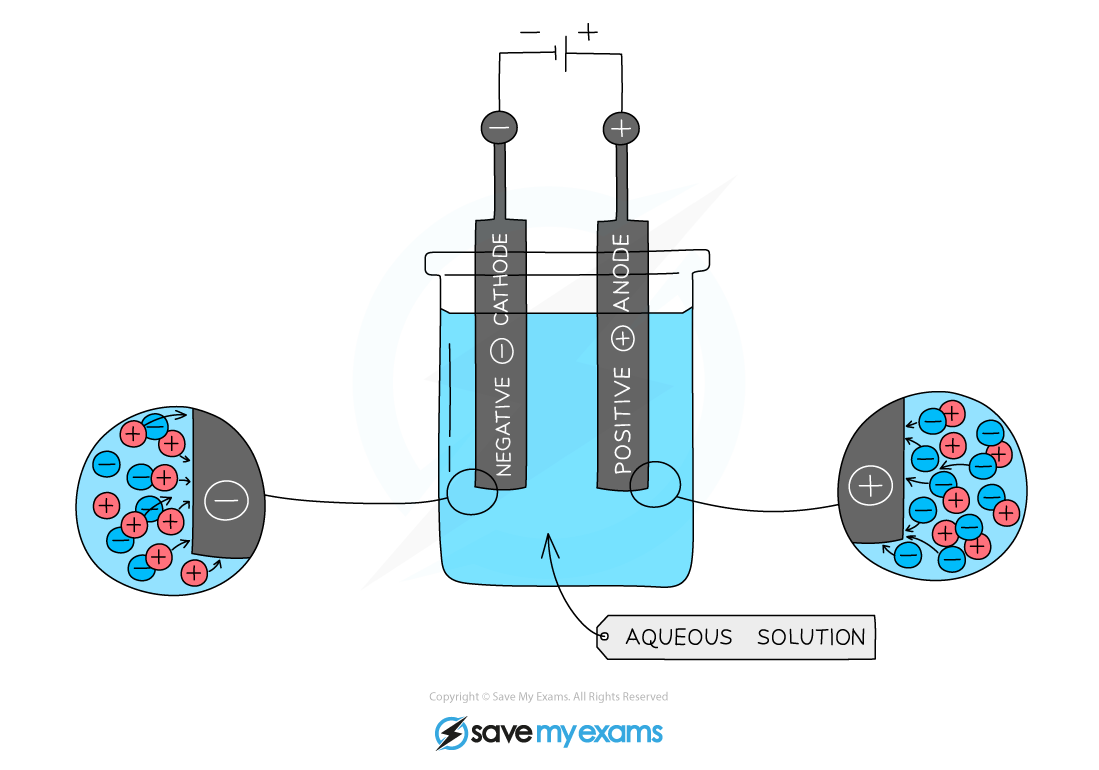 Set up of electrolysis
Set up of electrolysis
- An example which is simple to see the separation is using copper(II) chromate(VI), CuCrO4
- The solution contains
- Cu2+ ions (blue)
- CrO42- ions (yellow)
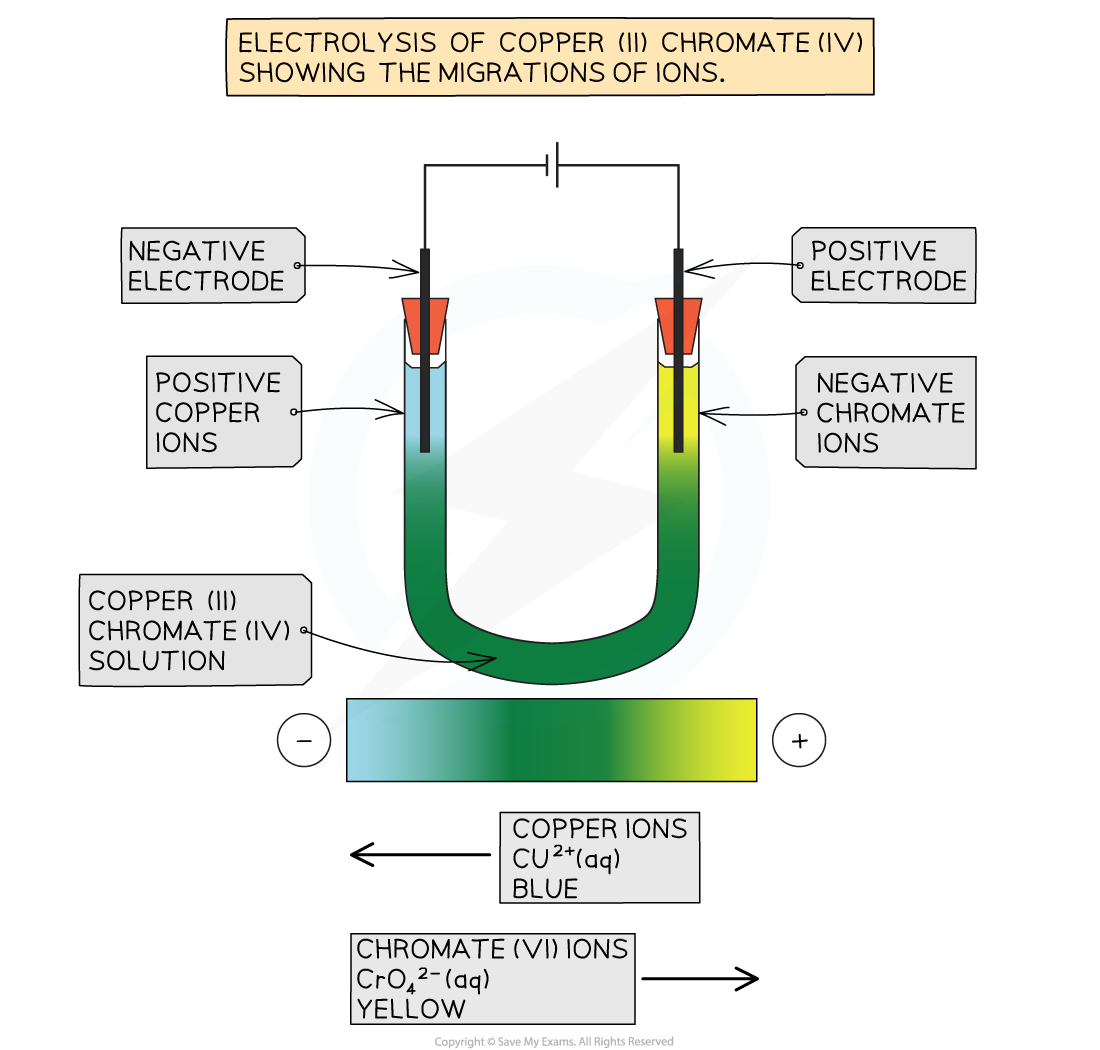
- Overall the solution is an olive green colour, but when the solution undergoes electrolysis a blue colour appears around the negative electrode, and a yellow colour appears around the positive electrode
- This is because the Cu2+ ions are attracted to the negative electrode and their blue colour is observed and the CrO42- ions are attracted to the positive electrode and their yellow colour is observed
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





