- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.4.3 Ionic Trends
Ionic Radius
Ionic radius
- The ionic radius of an element is a measure of the size of an ion
- Ionic radii show predictable patterns
- Ionic radii increase with increasing negative charge
- Ionic radii decrease with increasing positive charge
- For negative ions
- They are formed by atoms gaining electrons
- The outermost electrons are further away from the positively charged nucleus and are therefore held only weakly to the nucleus which increases the ionic radius
- The greater the negative charge, the larger the ionic radius
- For positive ions
- Positively charged ions are formed by atoms losing electrons
- The nuclear charge remains the same but there are now fewer electrons which undergo a greater electrostatic force of attraction to the nucleus which decreases the ionic radius
- The greater the positive charger, the smaller the ionic radius
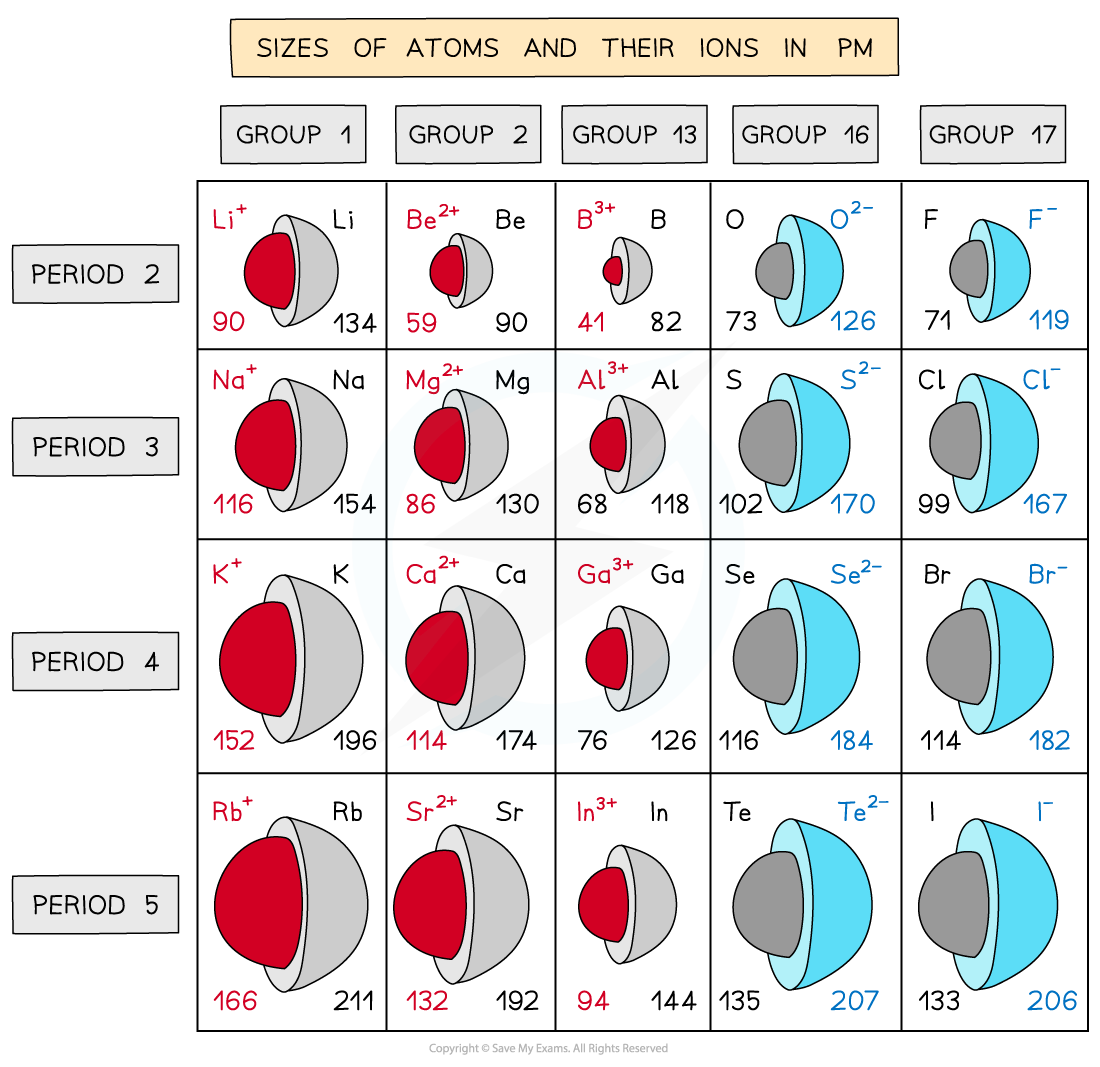
Trends in the ionic radii across a period and down a group
Isoelectronic Ions
- These are ions that have the same electronic configuration
- For example the following ions all have the electronic configuration of 1s2 2s2 2p6
- However, they all have different numbers of protons, therefore will have a different ionic radius
- N3- (7 protons)
- O2- (8 protons)
- F- (9 protons)
- Na+ (11 protons)
- Mg2+ (12 protons)
- Al3+ (13 protons)
- As the number of protons in the nucleus of the ion increases, the electrons get pulled in more closely to the nucleus
- The radii of the isoelectronic ions therefore fall across this series of ions
- N3- has an ionic radius of 0.171 nm and Al3+ has an ionic radius of 0.054 nm
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





