- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.2.2 Quantum Shells
Developing Electronic Configuration
- Electrons move rapidly around the nucleus in energy shells
- If their energy is increased, then they can jump to a higher energy level
- The process is reversible, so electrons can return to their original energy levels
- When this happens, they emit energy
- The frequency of energy is exactly the same, it is just being emitted rather than absorbed:
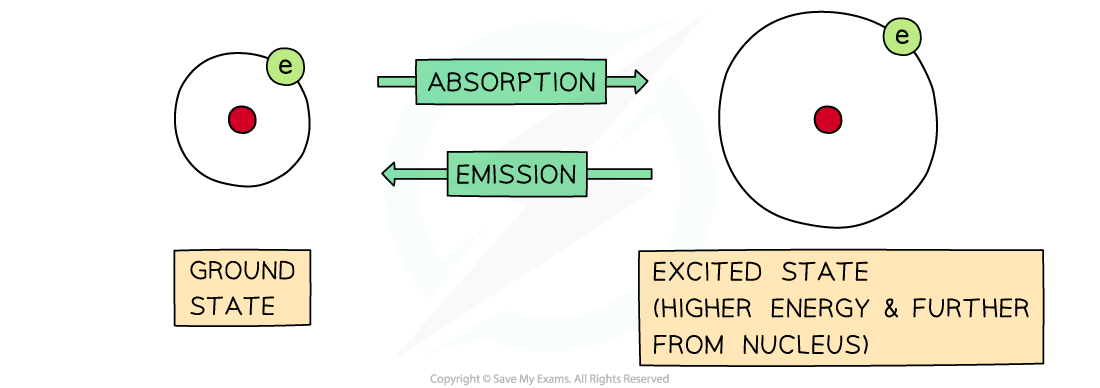
The difference between absorption and emission depends on whether electrons are jumping from lower to higher energy levels or the other way around
- The energy they emit is a mixture of different frequencies
- This is thought to correspond to the many possibilities of electron jumps between energy shells
- If the emitted energy is in the visible region, it can be analysed by passing it through a diffraction grating
- The result is a line emission spectrum
Line emission spectra

The line emission (visible) spectrum of hydrogen
- Each line is a specific energy value
- This suggests that electrons can only possess a limited choice of allowed energies
- These packets of energy are called 'quanta' (plural quantum)
- What you should notice about this spectrum is that the lines get closer together towards the blue end of the spectrum
- This is called convergence and the set of lines is converging towards the higher energy end, so the electron is reaching a maximum amount of energy
- This maximum corresponds to the ionisation energy of the electron
- These lines were first observed by the Swiss school teacher Johannes Balmer, and they are named after him
- We now know that these lines correspond to the electron jumping from higher levels down to the second or n = 2 energy level
Successive ionisation energies
- The first electron removed has a low IE1 as it is easily removed from the atom due to the spin-pair repulsion of the electrons in the 4s orbital
- The second electron is more difficult to remove than the first electron as there is no spin-pair repulsion
- The third electron is much more difficult to remove than the second one corresponding to the fact that the third electron is in a principal quantum shell which is closer to the nucleus (3p)
- Removal of the fourth electron is more difficult as the orbital is no longer full, and there is less spin-pair repulsion
- The graph shows there is a large increase in successive ionisation energy as the electrons are being removed from an increasingly positive ion
- The big jumps on the graph show the change of shell and the small jumps are the change of subshell
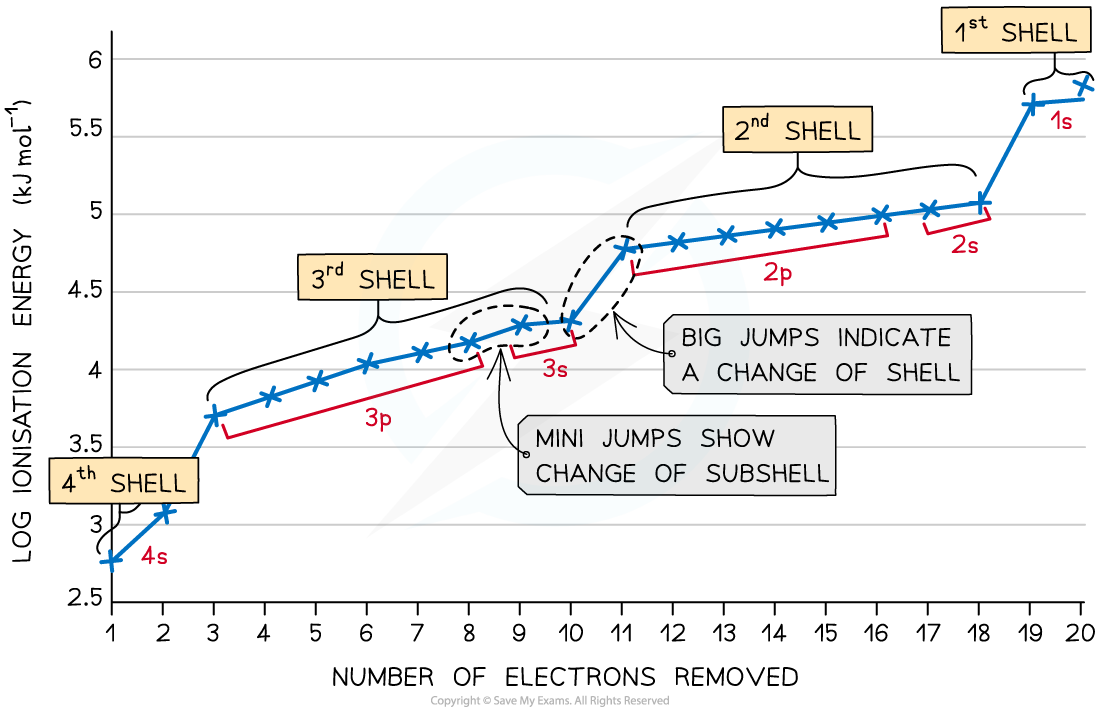
Successive ionisation energies for the element calcium
Filling Quantum Shells
Shells
- The arrangement of electrons in an atom is called the electron configuration
- Electrons are arranged around the nucleus in principal energy levels or principal quantum shells
- Principal quantum numbers (n) are used to number the energy levels or quantum shells
- The lower the principal quantum number, the closer the shell is to the nucleus
- So, the first shell which is the closest to the nucleus is n = 1
- The higher the principal quantum number, the greater the energy of the shell and the further away from the nucleus
- The lower the principal quantum number, the closer the shell is to the nucleus
- Each principal quantum number has a fixed number of electrons it can hold
- n = 1 : up to 2 electrons
- n = 2 : up to 8 electrons
- n = 3 : up to 18 electrons
- n = 4 : up to 32 electrons
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





